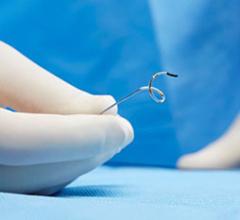
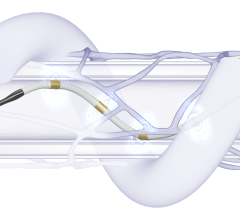
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) procedure, a minimally invasive treatment now available at Long Beach Medical ...
Hypertension
This page includes news and new technology on hypertension. The condition is caused by abnormally high blood pressure that causes excess force and pressure of the blood against the artery walls. Long-term, this can cause damage the vessels and contributes to heart disease. Therapies include antihypertensive medications such as diurestics and renal denervation therapy.
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
Oct. 30, 2025 — Recor Medical and its parent company, Otsuka Medical Devices presented the results from two clinical ...

Hypertension remains the leading modifiable driver of cardiovascular disease and premature death.1 Even with multiple ...
Sept. 04, 2025 — CorVista Health has published a new case series in the Journal of the American College of Cardiology ...
Sept. 5, 2025 — Idorsia Ltd has announced a first-of-its-kind initiative with the Stanford Hypertension Center and Duke ...
Aug. 19, 2025 — Idorsia Ltd recently announced its novel dual endothelin receptor antagonist (ERA), Tryvio (aprocitentan ...
July 7, 2025 — Medtronic announced that it has treated the first patient in its SPYRAL GEMINI Pilot program (including ...
May 19, 2025 — Gradient Denervation Technologies recently announced the company’s pulmonary denervation system has ...
April 9, 2025 — Idorsia Ltd recently announced that the US Food & Drug Administration (FDA), after having released ...
Mar. 10, 2025-- Corcept Therapeutics Inc., a commercial-stage company engaged in the discovery and development of ...
At the Cardiovascular Research Technologies (CRT) 2025 conference in Washington, D.C., Medtronic presented new data and ...
Jan. 13, 2025 — Medtronic recently announced that the Centers for Medicare & Medicaid Services (CMS) is opening a ...
Jan. 9, 2025 — Gender-based racism through microaggressions may be linked to higher blood pressure postpartum and beyond ...
Jan. 13, 2025 — Medtronic plc recently announced that the Centers for Medicare & Medicaid Services (CMS) is opening a ...
Jan. 8, 2025 — Mineralys Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing medicines ...




 January 19, 2026
January 19, 2026