April 30, 2020 — A large study in adolescents and children, some as young as 3 years of age, shows a link between ...
Hypertension
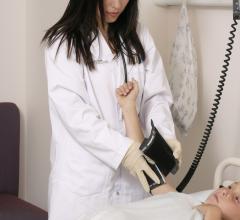
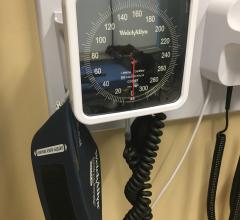
This page includes news and new technology on hypertension. The condition is caused by abnormally high blood pressure that causes excess force and pressure of the blood against the artery walls. Long-term, this can cause damage the vessels and contributes to heart disease. Therapies include antihypertensive medications such as diurestics and renal denervation therapy.
April 8, 2020 — There has been debate in the past month or so during the novel coronavirus (COVID-19) pandemic whether ...
April 1, 2020 — Three months after undergoing renal denervation (RDN), patients with untreated high blood pressure had ...
April 1, 2020 — Three months after undergoing renal denervation (RDN), patients with untreated high blood pressure had ...
March 16, 2020 — The European Society of Cardiology (ESC) issued a statement March 13 recommending in novel coronavirus ...
February 27, 2020 — Aria CV Inc. completed a $31 million Series B round of financing to fund its first clinical study in ...
February 7, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the Aria ...
October 10. 2019 — Late-breaking results from its MODERATO II double-blind, randomized study of BackBeat Cardiac ...
August 20, 2019 — In a study that spanned two and a half decades and looked at data from more than 4,700 participants ...
August 19, 2019 — The U.S. Food and Drug Administration (FDA) granted market clearance the Barostim Neo System for the ...
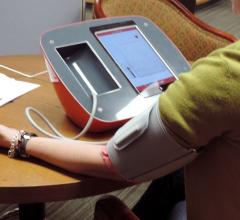
August 14, 2019 — People enrolled in a large clinical hypertension management trial were half as likely to control their ...
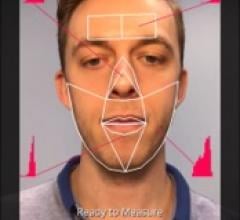
August 6, 2019 — Blood pressure monitoring might one day become as easy as taking a video selfie, according to new ...
July 29, 2019 — High blood pressure among children is on the rise and a lack of research about how to treat it has left ...
July 16, 2019 — Elevated blood pressure and cholesterol levels in young adulthood may lead to an increased risk of heart ...
July 3, 2019 — The American College of Cardiology (ACC) has partnered with Veradigm, an Allscripts business unit, to ...

 April 30, 2020
April 30, 2020