Jan. 13, 2025 — Medtronic recently announced that the Centers for Medicare & Medicaid Services (CMS) is opening a national coverage analysis (NCA) on renal denervation, a process that will allow the agency to review and develop a national Medicare coverage policy for renal denervation procedures for patients with hypertension. This national coverage analysis was initiated by CMS in response to Medtronic's request to support Medicare beneficiary access to the Symplicity Spyral renal denervation (RDN) system, used in the Symplicity blood pressure procedure.
"Hypertension is a global health epidemic that impacts a wide variety of patients," said Jason Weidman, senior vice president and president of the Coronary and Renal Denervation business within the Cardiovascular Portfolio at Medtronic. "As the leader in developing a minimally invasive treatment option for hypertension, Medtronic has been closely engaged with CMS to establish a national coverage pathway for Symplicity Spyral. We appreciate CMS' efforts in creating new pathways to expedite access to breakthrough technologies like Symplicity Spyral and look forward to our continued partnership in developing a national coverage policy."
CMS' action follows Medtronic's work with the agency to pilot the framework for the Transitional Coverage for Emerging Technologies (TCET) pathway to establish coverage for the Symplicity Spyral renal denervation system, a U.S. FDA-approved breakthrough device. As referenced in the CMS tracking sheet, the expected completion date for the national coverage analysis is Oct. 11, 2025. Until a national coverage determination is put into effect, Symplicity blood pressure procedures will continue to be evaluated for coverage based on medical necessity for individual Medicare patients.
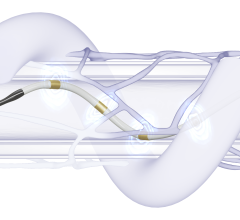
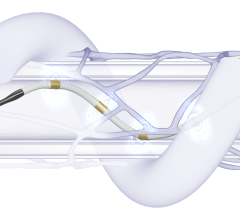
The Symplicity Spyral RDN system, approved by the U.S. Food and Drug Administration (FDA) in November 2023, is an innovative, minimally invasive procedure that delivers radiofrequency energy to nerves near the kidneys that can become overactive and contribute to high blood pressure. Symplicity Spyral is the only commercially available RDN device with the breadth of durable, consistent, long-term data and single catheter design. The SPYRAL-HTN clinical program has demonstrated 18 mmHg office blood pressure reductions out to three years in a real-world setting,1 has the longest and largest real-world registry2 and the largest dataset showing long-term reductions without the need for additional medication.2-3
The Medtronic SPYRAL HTN Global Clinical Program is the most comprehensive clinical program studying RDN in more than 4,000 patients in the presence and absence of medication, and with high baseline cardiovascular risk. The Symplicity blood pressure procedure has demonstrated sustained and durable drops in blood pressure out to three years in randomized control and real-world registry trials.5-8 The Symplicity RDN system is approved for commercial use in more than 75 countries around the world.
Hypertension, or high blood pressure, impacts more than1 billion adults worldwide, and is the leading modifiable cause of heart attack, stroke, and death.4 Despite available treatment with medications and lifestyle changes, blood pressure remains uncontrolled for many patients. Nearly 80% of adults with hypertension do not have it under control1 and half of hypertension patients become non-adherent to medication within one year.4-5
1 Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomized, sham-controlled trial. The Lancet. 2022; 399:1401-1410.
2 Mahfoud F, Mancia G, Schmieder RE, et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension. 2023 Aug ;80(8):1759-177.
3 Kandzari DE et al. Long-term Safety and Efficacy of Radiofrequency Renal Denervation in the Presence of Antihypertensive Drugs: 24-Month Results from the SPYRAL HTN–ON MED Randomized Trial. TCT 2024
4 WHO. Hypertension fact sheet. September 13, 2019. Available at: https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed February 15, 2022.
5 Bhatt, D. et al, Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. The Lancet. September 18, 2022. DOI:https://doi.org/10.1016/S0140-6736(opens new window)(22)01787-1.
6 Mahfoud, F. et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension. August 2023; DOI: 10.1161/HYPERTENSIONAHA.123.21283.
7Mahfoud, F. et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. The Lancet. April 2022. https://doi.org/10.1016/S0140-6736(22)00455-X(opens new window)(opens new window)
8 Mahfoud, F. et al. Cardiovascular Risk Reduction After Renal Denervation According to Time in Therapeutic Systolic Blood Pressure Range. Journal of the American College of Cardiology. November 2022. https://doi.org/10.1016/j.jacc.2022.08.802


 January 19, 2026
January 19, 2026