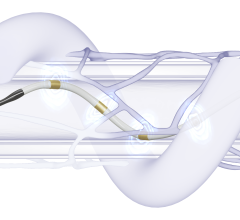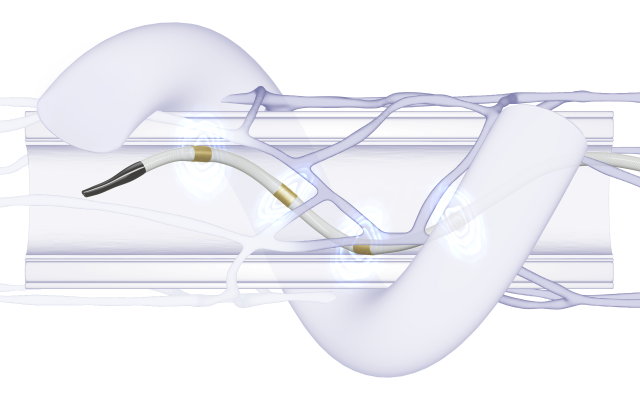
Jan. 13, 2025 — Medtronic plc recently announced that the Centers for Medicare & Medicaid Services (CMS) is opening a national coverage analysis (NCA) on renal denervation, a process that will allow the agency to review and develop a national Medicare coverage policy for renal denervation procedures for patients with hypertension. This national coverage analysis was initiated by CMS in response to Medtronic's request to support Medicare beneficiary access to the Symplicity Spyral renal denervation (RDN) system, used in the Symplicity blood pressure procedure.
"Hypertension is a global health epidemic that impacts a wide variety of patients," said Jason Weidman, senior vice president and president of the Coronary and Renal Denervation business within the Cardiovascular Portfolio at Medtronic. "As the leader in developing a minimally invasive treatment option for hypertension, Medtronic has been closely engaged with CMS to establish a national coverage pathway for Symplicity Spyral. We appreciate CMS' efforts in creating new pathways to expedite access to breakthrough technologies like Symplicity Spyral and look forward to our continued partnership in developing a national coverage policy."
CMS' action follows Medtronic's work with the agency to pilot the framework for the Transitional Coverage for Emerging Technologies (TCET) pathway to establish coverage for the Symplicity Spyral renal denervation system, a U.S. FDA-approved breakthrough device. As referenced in the CMS tracking sheet, the expected completion date for the national coverage analysis is October 11, 2025. Until a national coverage determination is put into effect, Symplicity blood pressure procedures will continue to be evaluated for coverage based on medical necessity for individual Medicare patients.
For more information on Medtronic, visit www.Medtronic.com


 January 19, 2026
January 19, 2026