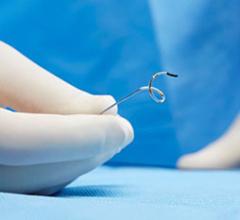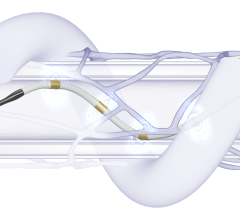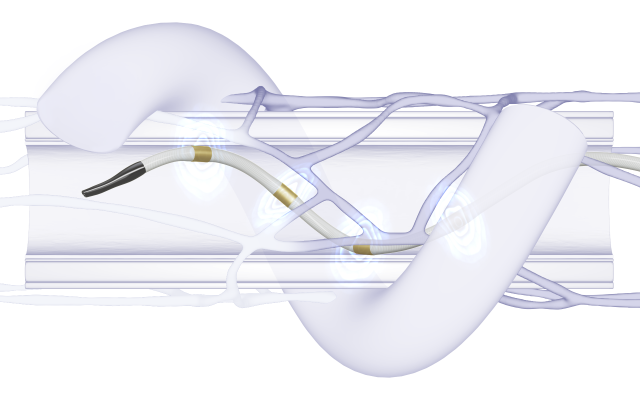
During the Symplicity Spyral procedure, radiofrequency energy is applied to ablate the sympathetic nerve fibers surrounding the renal arteries. All Photos: Medtronic
Hypertension remains the leading modifiable driver of cardiovascular disease and premature death.1 Even with multiple drug classes available, nearly 70% of adults have uncontrolled blood pressure.2
The August update to the American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines addresses this gap by including renal denervation (RDN) as a Class IIb recommendation for the first time.3 RDN may be considered as an adjunctive therapy alongside lifestyle modification and medication for carefully selected adults who have resistant or uncontrolled hypertension.
The new guidance follows a similar update to the 2023 European Society of Hypertension guidelines recommending RDN as a third-level treatment for hypertension.4
“Hypertension accounts for billions in avoidable costs each year, much of it tied to complications from uncontrolled blood pressure and medication nonadherence,” says Jason Fontana, vice president and general manager of RDN for Medtronic. “By providing an always-on therapy that complements existing treatments, RDN has the potential to improve long-term outcomes while helping health systems reduce the downstream burdens of cardiovascular complications.”
An Emerging Therapy

Developed in the early 2000s, RDN is a minimally invasive technique performed using either ultrasound or radiofrequency energy. Both approaches have been used in Europe since receiving CE approvals in 20126 and 2013 and were approved for use in the United States by the FDA in November 2023.7
One of the most tested devices is the Symplicity Spyral RDN System from Medtronic, used in more than 25,000 patients worldwide.8 It combines a spiral multielectrode catheter with a radiofrequency generator. Advanced via femoral access, the catheter delivers controlled energy to ablate sympathetic nerves in the renal artery.
Across clinical trials, Symplicity has demonstrated mean office systolic blood pressure (SBP) reductions of about 9 mm/Hg at three to six months, both in patients on and off medications.9-10 It has also achieved a sustained 17 mm/Hg mean reduction in office SBP real-world patients at three years.11
“RDN works about 70% of the time with a 5mm/Hg ambulatory SBP reduction, which translates to a 9 or 10 mm/Hg [in-office] SBP reduction,” says Raymond Townsend, MD, a nephrologist and professor of medicine at the University of Pennsylvania, who has published 50 publications in the RDN domain. “When it works, you don’t have to repeat it, and it doesn’t have all the side effects that drugs have.” Risks of the procedure are similar to the risks of other procedures involving arterial catheterization.12
Patient eligibility
Per the AHA/ACC guidelines, RDN may be reasonable for patients who have resistant hypertension (office SBP 140mm to 180 mm/Hg and diastolic blood pressure ≥90 mm/Hg and eGFR ≥40 mL/min/1.73 m2). The ideal candidate, says Dr. Townsend, is a patient using two antihypertensive medications faithfully without success who is facing either adding a third drug or increasing their current dosage toward their maximum levels. A small group of patients who are on five or six antihypertensive medications may also benefit.

“RDN is not a substitute for medical therapy,” Dr. Townsend says. “Patients probably will not get off of any medications afterward. But they’re doing this because they are 10 to 20 points above their current target. If it works and gives you a 10-to-15-point SBP reduction, that gets you a lot closer to and sometimes at goal, but still in the presence of medication.”
When counseling patients, Dr. Townsend advises cardiologists and interventional cardiologists to explain the potential risks and manage expectations. Most patients, he says, will see a modest but definite SBP reduction at three to six months, then see additional improvement over the next 30 months.
“It’s true in Medtronic trials that once you have a person who’s at least 5 mm/Hg lower at three or six months, odds are they’re going to be 7 mm/Hg lower at one year, 8 or 9 mm/Hg lower at two years, and 9, 10, or 11 mm/Hg lower at three years.”
Medtronic’s Fontana says RDN is just the start of potential advances aimed at helping patients and providers manage hypertension better. “We have some exciting next-generation programs and are studying multiorgan denervation in the kidneys and liver to see the possible impact on hypertension,” he says.
References
1. World Health Organization. Hypertension. Published Sept. 16, 2025. Accessed Sept. 29, 2025. https://www.who.int/news-room/fact-sheets/detail/hypertension
2. Gupta A, Spertus JA, Turakhia M, et al. Efficacy of Renal Denervation in Resistant Hypertension: The RADIANCE II Randomized Clinical Trial. JAMA Netw Open. 2025;8(5):e2292801. doi:10.1001/jamanetworkopen.2022.92801
3. Jones, D.W. M.D. et al, 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. August 2025. Table 25. https://doi.org/10.1161/CIR.0000000000001356
4. Mahfoud F, Wijns W. Renal Denervation for Treatment of Hypertension: From High-Level Quality Evidence to Implementation in Clinical Practice. J Soc Cardiovasc Angiogr Interv. 2023;2(6Part A):101130. Published Aug. 23, 2023.
5. Fengler K. Renal Denervation for Resistant Hypertension: A Concise Update on Treatment Options and the Latest Clinical Evidence. Cardiol Ther. 2022;11(3):385-392. https://doi:10.1007/s40119-022-00275-5
6. Endovascular Today. Recor Medical Receives CE Mark Approval for Paradise Renal Denervation System. Published Nov. 14, 2024. Accessed Sept. 29, 2025. https://evtoday.com/news/recor-medical-receives-ce-mark-approval-for-paradise-renal-denervation-system
7. Zhang J, Belford PM, Stouffer GA. Renal Denervation After USA FDA Approval: An Update from an Interventional Cardiologist’s Perspective. J Clin Med. 2025;14(10):3554. Published May 19, 2025. doi:10.3390/jcm14103554
8. Medtronic. Symplicity Spyral Renal Denervation System. Updated 2025. Accessed Sept. 29, 2025. www.medtronic.com/en-us/healthcare-professionals/products/surgical-energy/ablation/radiofrequency-ablation/systems/symplicity-spyral-renal-denervation-system.html
9. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451. doi: 10.1016/S0140-6736(20)30554-7.
>10. Kandzari D, Townsend R, Kario K, et al. Safety and efficacy of renal denervation in patients taking antihypertensive medications. J Am Coll Cardiol. 2023;82(19):1809–1823. doi: 10.1016/j.jacc.2023.08.045
11. Mahfoud F, Mancia G, Schmieder RE, et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension. 2023;80(8):1759-1770. doi:10.1161/HYPERTENSIONAHA.123.21283
12. Medtronic. Potential Risks of Renal Denervation (RDN). Updated 2025. Accessed Sept. 29, 2025. https://www.medtronic.com/in-en/patients/treatments-therapies/hypertension-high-blood-pressure/about-rdn/potential-risks.html


 January 19, 2026
January 19, 2026