August 16, 2021 — The U.S. Food and Drug Administration (FDA) approved Abbott's Amplatzer Amulet Left Atrial Appendage ...
Antiplatelet and Anticoagulation Therapies
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, international normalized ratio (INR) testing, oral anticoagulants, IV administered drugs such as Heprin, and dual antiplatelet therapy (DAPT). The most commonly used anticoagulant is warfarin, which available in generic form at a low cost. However, it has a narrow therapeutic window and its effectiveness is altered by food containing vitamin K. To regulate warfarin, regular INR testing is needed. The newer anticoagulation drugs are referred to as novel oral anticoagulant (NOAC). However, since some of these drugs are now more than six years old, they are commonly referred to as non-vitamin K oral anticoagulant (NOAC), and the newest term, direct oral anticoagulant (DOAC). NOAC or DOAC agents have much larger therapeutic windows, do not require INR testing. Aspirin and clopidogrel (Plavix) are the most commonly used antiplatelet agents for the prevention of heart attacks and stroke. They are often prescribed together as DAPT.
July 29, 2021 – Results of the STROKE-VT trial shows direct oral anticoagulants (DOACs) are more effective than aspirin ...
July 15, 2021 — COVID-19 is marked by heightened inflammation and abnormal clotting in the blood vessels, particularly ...
June 30, 2021 — Abbott announced its Xience family of drug-eluting coronary stents received U.S. Food and Drug ...
June 24, 2021 — Data captured in American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) regis ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
May 17, 2021 — The anticoagulant rivaroxaban (Xarelto), in addition to low-dose aspirin, significantly reduced the ...
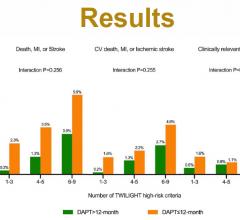
May 15, 2021 — The ADAPTABLE trial found no significant differences in cardiovascular events or major bleeding in ...
May 15, 2021 — The anticoagulant apixaban (Eliquis) was not superior to standard of care following transcatheter aortic ...
April 28, 2021 — An analysis of the prospective Chinese Fuwai PCI Registry, confirms long-term, dual-antiplatelet ...
April 6, 2021 — Abbott today announced its Xience stent has received CE mark in Europe for shorter duration of dual anti ...
Behnood Bikdeli M.D., a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, offers an ...
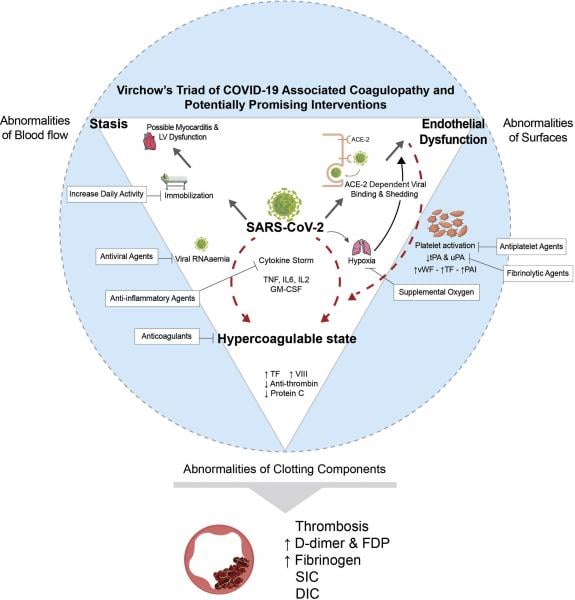
A comprehensive review or more than 80 randomized controlled trials (RCTs) investigating how to best manage optimal ...

December 23, 2020 — Three clinical trial platforms working together to test the effects of full doses of anticoagulants ...
November 18, 2020 — The primary results from the RIVER Trial, Rivaroxaban for Valvular Heart disease and Atrial ...


 August 16, 2021
August 16, 2021






![Comparison showing platelet adhesion to the surface of various coronary artery drug-eluting stents (DES) in a preclinical study that used aspirin only. Abbott said the Xience stent's fluoropolymer is significantly more anti-thrombotic than other DES.[2]](/sites/default/files/styles/content_feed_medium/public/DES_Comparison_thrombus_formation_Stents_Abbott.jpg?itok=mfh9GUz-)

