
Thrombus formation in the aortic arch in a 46-year-old COVID patients in the ICU. Three trials are underway to find which anticoagulant strategy is best to treat moderate and critically ill patients where COVID-causes venous thrombo-embolism (VTE) is a major cause of complications. Image courtesy of Margarita Revzin et al.
December 23, 2020 — Three clinical trial platforms working together to test the effects of full doses of anticoagulants in COVID-19 patients have paused enrollment for one group of patients. Among critically ill COVID-19 (SARS-CoV-2) patients requiring intensive care unit (ICU) support, therapeutic anticoagulation drugs did not reduce the need for organ support. Enrollment continues for moderately ill hospitalized COVID-19 patients in the trials.
As is normal for clinical trials, these trials are overseen by independent boards that routinely review the data and are composed of experts in ethics, biostatistics, clinical trials, and blood clotting disorders. Informed by the deliberations of these oversight boards, all the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19. A potential for harm in this sub-group could not be excluded. Increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses that will be made available as soon as possible.
At the recommendation of the oversight boards, patients who do not require ICU care at the time of enrollment will continue to be enrolled in the trial. Whether the use of full-dose compared to low-dose blood thinners leads to better outcomes in hospitalized patients with less severe COVID disease remains a very important question. Patients who require full-dose blood thinners for another medical indication are not included in these trials.
COVID Causes Widespread Blood Clots, Leading to Serious Complications
COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. This venous thrombosis embolism (VTE) can cause stroke, heart attacks, pulmonary embolism (PE), deep vein thrombosis (DVT) and organ failure. These NIH trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels.
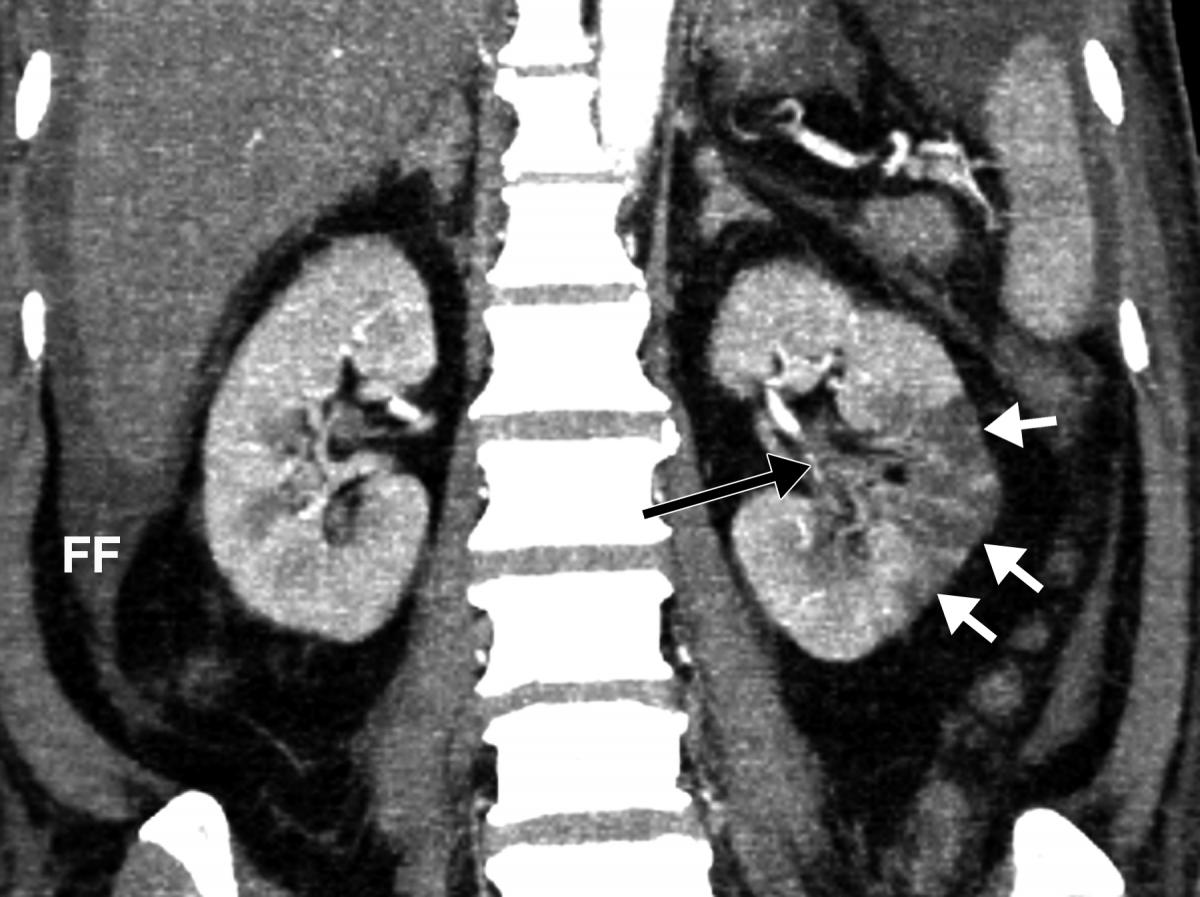
Example of kidney infarct organ damage caused by COVID-caused clotting in a 57-year-old man with COVID-19 who presented with abdominal pain. Image courtesy of Margarita Rezvin et al.
Three Trials Looking at Anticoaguation Strategies to Prevent COVID Bloot Clots
Three international partners have come together in an unprecedented collaboration resulting in a multiple platform randomized controlled trial. The three international trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials which span four continents have the common goal of assessing the benefit of full doses of blood thinners to treat moderately ill or critically ill adults hospitalized for COVID-19, compared to a lower dose often used to prevent blood clots in hospitalized patients. To meet the challenge of this pandemic, investigators worldwide joined forces to answer this question as rapidly as possible. In the United States, the ACTIV-4 trial is being led by a collaborative effort with several universities, including the University of Pittsburgh and New York University, New York City.
The trials are supported by multiple international funding organizations including the Canadian Institutes of Health Research, the National Institute for Health Research (U.K.), the National Health and Medical Research Council (Australia), the National Institutes of Health (U.S.), and the PREPARE and RECOVER consortia (EU).
About the National Heart, Lung, and Blood Institute (NHLBI): NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information: www.nhlbi.nih.gov.


 August 28, 2023
August 28, 2023 









