EchoPixel showed technology at TCT 2019 that creates live holograms in the cath lab from 3-D TEE imaging. It projects the holograms on a special display screen that does not require the user to wear 3-D glasses. The interventional cardiologist can use hand movements and a foot switch to move the image around without breaking the sterile field. It offers a new way to visualize catheters, device positioning and deployment in structural heart procedures. (Photos by Dave Fornell)
The latest in interventional cardiology clinical data and new device technologies were highlighted at the annual Transcatheter Cardiovascular Therapeutics (TCT) conference. This is an overview of some of the biggest takeaways from TCT 2019. The hottest topics included:
• Short duration dual-antiplatelet therapy (DAPT) using newer generation DES;
• Safety of paclitaxel coated devices;
• New TAVR devices seeking entry into the U.S. market;
• New COAPT Trial data on MitraClip use in heart failure patients;
• CT study of TAVR valve leaflet thrombosis; and
• Potential game-changing technologies on the expo floor.
Potential Game-changing Interventional Technologies on the Expo Floor
There were two very innovative new technologies shown for the first time on the expo floor at TCT 2019. One converts live transesophageal echo (TEE) imaging into real-time holographic video in the cath lab to aid structural heart procedural guidance. The second allows interventional cardiologists to virtually remodel blocked coronary arteries from computed tomography (CT) scans to help determine if revascularization will help a patient and to pre-plan the procedure.
EchoPixel showed technology that creates live holograms in the cath lab from 3-D TEE imaging. It projects the image as true 3-D holograms on a special display screen that does not require the user to wear 3-D glasses. The interventional cardiologist can use hand movements and a foot switch to move the image around without breaking the sterile field. It allows the operator the ability to see catheters or devices being moved in real time in a 3-D format. The system is intuitive to use so anyone can learn it in a couple minutes. It was submitted for FDA 510(k) regulatory review in September 2019.
The vendor is collaborating with GE Healthcare to interface the technology with the E95 system, but EchoPixel developed its own API for potential interfaces with any vendor. The company is approaching other imaging system vendors and an electrophysiology vendor for possible collaborations.
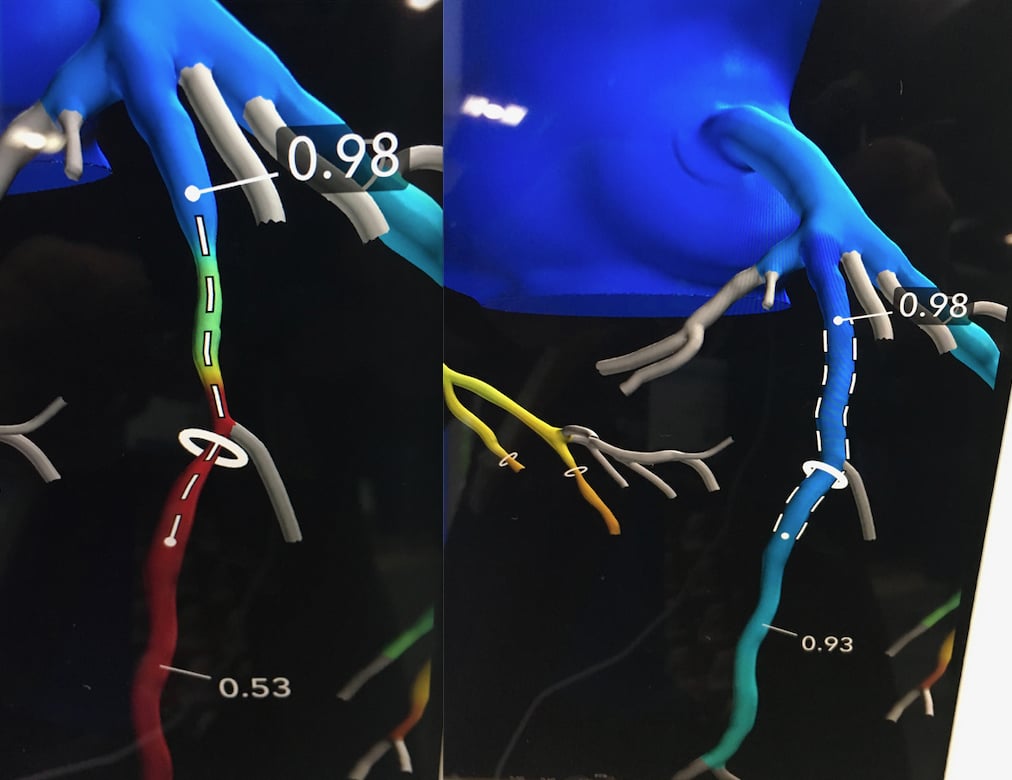
An example of the HeartFlow Planner software offers a noninvasive, real-time virtual modeling tool for coronary artery disease (CAD) intervention. It enables interventional cardiologists to virtually model clinical scenarios vessel-by-vessel on a 3-D coronary tree that color-code maps the fractional-flow reserve-computed tomography (FFR-CT) values for each vessel.
HeartFlow showed an interesting new tool called the HeartFlow Planner. It is a a noninvasive, real-time virtual modeling tool for coronary artery disease (CAD) intervention. It enables interventional cardiologists to virtually model clinical scenarios vessel-by-vessel on a 3-D coronary tree that color-code maps the fractional-flow reserve-computed tomography (FFR-CT) values for each vessel. It is an advancement in HeartFlow's FFR-CT technology, which takes CT heart scans and calculates virtual FFR values for coronary flow through a supercomputing, computational fluid dynamics algorithm.
The planner software allows operators to explore treatment strategies for patients with CAD before each procedure, review cases with colleagues and ensure everyone has a clear picture of the initial treatment plan. The user chooses a vessel segment to open to the fullest extent of the native vessel lumen and the software recalculates the FFR-CT values.
The software does not take into account heavy calcium, existing stents or other issues that might be encountered, but gives the operator an idea of which lesions are critical to the patient.
The vendor received FDA clearance for the HeartFlow Planner software in September 2019.
Shorter DAPT Using New Generation DES
The newest generations of drug-eluting stents (DES) have much better vessel healing and faster endothelialization profiles than earlier generation DES. This has removed the late-stent thrombosis issues with earlier generation stents that required 12 months or more of dual-antiplatelet therapy as a preventative protection measure for the patient. This has raised the question if DAPT duration after DES implantation can be shortened for patients who are at high-risk for bleeding complications caused by the blood thinners. Another area of research with newer DES is the use of monotherapy with newer antiplatelet agents and removing aspirin. Both of these strategies had positive results in six late-breaking trial presentations at TCT 2019.
 "Fundamentally what we have seen over the past few years is this movement establish individual patient risk — risk of an ischemic event and risk of a bleeding event — and then we want to try and balance those two things with the combination of antiplatelet and or anticoagulant therapies and how long do you go," said American Heart Association (AHA) President Robert Harrington, M.D., interventional cardiologist and the Arthur L. Bloomfield Professor of Medicine and chairman of the Department of Medicine at Stanford University.
"Fundamentally what we have seen over the past few years is this movement establish individual patient risk — risk of an ischemic event and risk of a bleeding event — and then we want to try and balance those two things with the combination of antiplatelet and or anticoagulant therapies and how long do you go," said American Heart Association (AHA) President Robert Harrington, M.D., interventional cardiologist and the Arthur L. Bloomfield Professor of Medicine and chairman of the Department of Medicine at Stanford University.
He said while stents have improved and are less thrombogenic, the newer antiplatelet agents ticagrelor and prasugrel are also better than the old standby of clopidogrel. This has led to the idea of peeling back the use of aspirin in high risk patients using the newer agents.
"Aspirin causes bleeding, in particular it causes mucous bleeding, GI bleeding and urinary bleeding," Harrington said. "There have now been several clinical trials, including the trials we saw at TCT, where we are starting to see the aspirin being removed and leaving the ADP inhibitor alone. This has led to less bleeding and a maintenance of the ischemic benefit."
Watch an interview with Harrington in the VIDEO: Overview of Short DAPT in High-risk Bleeding Patients Who Receive Stents.
The biggest of the short DAPT studies at TCT was the 9,006-patient TWILIGHT Trial, which looked at the use of ticagrelor monotherapy vs. ticagrelor with aspirin beginning at three months post percutaneous coronary intervention (PCI).
 "What we found was ticagrelor monotherapy was associated with a 45 percent reduction in clinically significant bleeds and absolutely no difference in death or myocardial infarction," said Roxana Mehran, M.D., trial principal investigator, and director of interventional cardiovascular research and clinical trials at the Zena and Michael A. Wiener Cardiovascular Institute at Mount Sinai School of Medicine.
"What we found was ticagrelor monotherapy was associated with a 45 percent reduction in clinically significant bleeds and absolutely no difference in death or myocardial infarction," said Roxana Mehran, M.D., trial principal investigator, and director of interventional cardiovascular research and clinical trials at the Zena and Michael A. Wiener Cardiovascular Institute at Mount Sinai School of Medicine.
Watch the VIDEO: TWILIGHT Trial Shows Benefit to Ticagrelor Monotherapy After Stent Implantation — an interview with Mehran.
Data from the 2,000-patient EVOLVE Short DAPT study found that shortened three-month DAPT in 1,493 patients did not increase myocardial infarction or stent thrombosis in high bleeding risk patients treated with a Boston Scientific Synergy DES. The stent uses a bioresorbable polymer drug carrier that completely dissolves within four months, leaving behind a bare metal stent.
"We found there were only four patients who had stent thrombosis, or a rate of 0.3 percent from three to 15 months, and the rate of myocardial infarction was similar to historical controls," said lead investigator Ajay J. Kirtane, M.D., director of the cardiac catheterization laboratories at NewYork-Presbyterian Hospital.
Learn more about the trial in an interview with Kirtane in the VIDEO: Early Discontinuation of DAPT in High Bleeding Risk Patients With the Synergy Stent.
The Onyx ONE Trial looked at use of just one month of DAPT and compared the Medtronic Onyx durable-polymer DES vs. the Biofreedom polymer-free drug-coated stent in patients at high risk of bleeding. The 1,996 patient study found that both are clinically safe and effective.
The IDEAL-LM Trial looked at four months of DAPT using the Synergy DES vs. 12-months of DAPT with the Abbott Xience DES. Synergy was found to be noninferior to 12 months of DAPT post-stent implantation among patients undergoing unprotected left main PCI.
The MODEL U-SES study from Japan looked at use of three-month DAPT in 1,695 patients at high bleeding risk treated with Terumo sirolimus-eluting Ultimaster stent, which uses a bioabsorbable polymer drug carrier. The study also looked at P2Y12 inhibitor monotherapy after the first three months compared with DAPT. The 1,695 patient study found a primary end point was a composite of death, infarction, stroke and definite or probable thrombosis bleeding at one year was 4.3 percent. It was found non-inferior compared to a similar patient cohort receiving one-year DAPT.
Safety Questions on Paclitaxel Coated Stents and Balloons
 The safety of paclitaxel eluting stents and drug-coated balloons (DCB) was first brought into question by a meta-analysis published in Journal of the American Heart Association in December 2018. That analysis showed a higher mortality rate at two years post-procedure and prompted the U.S. Food and Drug Administration (FDA) to release its first warning letter on the subject. At that time, the agency asserted that the benefits of paclitaxel-coated devices outweighed the possible risks when used as indicated, but more data was needed. In August 2019, the FDA released an updated MedWatch Alert after reviewing long-term follow-up clinical data. The agency said five-year results from three randomized trials showed an increased mortality rate in patients treated with these devices compared to those treated with uncoated devices. While these data provide reason for caution, the FDA noted that the devices still provide documented short-term benefits.
The safety of paclitaxel eluting stents and drug-coated balloons (DCB) was first brought into question by a meta-analysis published in Journal of the American Heart Association in December 2018. That analysis showed a higher mortality rate at two years post-procedure and prompted the U.S. Food and Drug Administration (FDA) to release its first warning letter on the subject. At that time, the agency asserted that the benefits of paclitaxel-coated devices outweighed the possible risks when used as indicated, but more data was needed. In August 2019, the FDA released an updated MedWatch Alert after reviewing long-term follow-up clinical data. The agency said five-year results from three randomized trials showed an increased mortality rate in patients treated with these devices compared to those treated with uncoated devices. While these data provide reason for caution, the FDA noted that the devices still provide documented short-term benefits.
A whole day of discussion at the TCT town hall meeting was devoted to presentations and debate about the safety of paclitaxel. Discussion centered on the clinical data to date, what is being done to gather more clinical information to prove or disprove any safety signals, and what hypothesis could be agreed on for how this signal appeared in the first place. No agreement could be found by the panel because there is no single issue identified as to what might be causing increased mortality.
Several experts on the panel discussion raised questions if this was an anomaly in the data caused by chance. Some suggested the data may be skewed by up to 25 percent of trial participants being lost to long-term follow-up and only the sickest patients remaining in the study cohorts.
Cardiovascular Research Foundation (CRF) CEO Juan F. Granada, M.D., who was heavily involved in DCB development, said he questions if there is an actual safety concern because there is no smoking gun connecting deaths across several trials to any particular cause. He said paclitaxel from the device does not appear to be causing any specific issues elsewhere in the body.
Others said the cause could be related to factors such as the combination of other drugs patients are taking and possible reactions with the paclitaxel. Others said it appears to be just a random coincidence, since there are no specific factors linking any of the deaths.
However, efforts are underway to gather additional data from Medicare to see if there are any patterns in care or drugs used in the patients who died. Also, questions have been added to new and ongoing trials for other paclitaxel-eluting devices that should offer more information from at least 16,000 patients in the next couple years.
One of the late-breaking trial presentations at TCT shed additional light on the issue. It was an independent analysis of Lutonix 035 DCB patient-level data showed no statistically significant mortality increase. This was one of the first major reviews of trial data for patients who were treated with a paclitaxel coated device used in peripheral vessels since the safety issue was first raised.
Read more about the LEVANT Five-Year Outcomes From Three Randomized Trials of Percutaneous Angioplasty With vs Without a Drug-Coated Balloon in Patients With Femoropopliteal Arterial Disease.
Read the article "FDA Panel Recommends Continued Use of Paclitaxel-coated Peripheral Devices."
New TAVR Devices Seeking U.S. FDA Approval
The biggest news in cardiovascular technology in 2019 was the August FDA clearance of the Edwards Lifesciences Sapien 3 and Medtronic CoreValve transcatheter aortic valve replacement (TAVR) devices in low-risk surgical patients. This opened TAVR use for all patients, allowing physicians to decide if TAVR or open heart surgery is best for the patient. Experts across the board expect surgical volumes to decline as TAVR values increase to about 75 percent of heart valve procedural volume by 2025.
This raises the bar for any new TAVR devices seeking FDA approval. The August FDA action did not include the third FDA-cleared TAVR valve, the Boston Scientific Lotus, because there is limited data on its use in lower risk patients. Two other devices are also seeking FDA market approval, the Abbott Portico and Boston Scientific's Acurate Neo, both of which had late-breaking trial presentations at TCT 2019, but left questions about their performance compared to the current Sapien and CoreValve systems.
 The SCOPE I Trial of the Acurate Neo versus the Sapien 3 in patients with severe aortic stenosis did not meet non-inferiority.
The SCOPE I Trial of the Acurate Neo versus the Sapien 3 in patients with severe aortic stenosis did not meet non-inferiority.
"The device was designed as a hybrid valve, blending the benefits of a self-expanding device with having specific annular contact with the aim of lowering pacemakers and stroke rates," explained Chandan Devireddy, M.D., cath lab director at Emory University Hospital Midtown, and a spokesman for the Society of Cardiovascular Angiography and Interventions (SCAI). "The data was not a happy story. It was designed as a non-inferiority trial, and it did not meet that. Instead it showed it was inferior against the Edwards Sapien valve."
He said the poorer outcomes were mainly driven by a higher rate of kidney injury, possibly from use of more contrast, and a high rate of paravalvular leak (PVL).
"Now we are in the third, if not the fourth, generation world of transcatheter devices. So that high a level of moderate PVL as seen in the Acurate Neo trial, it is a bitter pill to swallow. In 2019, the current generation of the Accurate Neo is not ready for prime time," Devireddy said.
He did note stroke rates were lower with the new device and that Boston Scientific has already developed a second generation device. The new version of the Accurate Neo valve has additional material at the base designed to reduce PVL.
 Data on the Portico FDA investigational device exemption (IDE) study were also presented at TCT 2019. The 30-day safety and one-year effectiveness outcomes of a self-expanding TAVR system for patients with severe aortic stenosis at high or extreme-risk for surgery was noninferior to contemporary FDA-approved TAVR systems. The study met both the pre-specified primary safety composite endpoint (all-cause mortality, disabling stroke, life threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, 13.8 vs 9.6 percent; non-inferiority = 0.03) and the primary effectiveness composite endpoint (all-cause mortality or disabling stroke at one-year, 14.9 vs 13.4 percent; non-inferiority = 0.006).
Data on the Portico FDA investigational device exemption (IDE) study were also presented at TCT 2019. The 30-day safety and one-year effectiveness outcomes of a self-expanding TAVR system for patients with severe aortic stenosis at high or extreme-risk for surgery was noninferior to contemporary FDA-approved TAVR systems. The study met both the pre-specified primary safety composite endpoint (all-cause mortality, disabling stroke, life threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, 13.8 vs 9.6 percent; non-inferiority = 0.03) and the primary effectiveness composite endpoint (all-cause mortality or disabling stroke at one-year, 14.9 vs 13.4 percent; non-inferiority = 0.006).
“By demonstrating results in line with contemporary TAVR systems and an improved delivery system, the Portico valve with FlexNav delivery system has demonstrated IDE clinical results on par with commercially available valves,” said Greg Fontana, M.D., trial principal investigator and director and chairman, cardiothoracic surgery, CardioVascular Institute of Los Robles Regional Medical Center.
While it did meet its thresholds for noninferiority, there were concerns about higher levels of moderate PVL, Devireddy said. He added Abbott is working on its second generation device, which will have a better annular sealing skirt.
Portico was developed at the same time as the first-generation Sapien and CoreValve devices, but the trial was stopped for a period after thrombus formation on the valve leaflets was found in CT imaging. This was a big, alarming news item at TCT a few years ago and led to reviews of other TAVR and surgical valves. It was found that thrombus formation is normal across all types of valves and usually dissipates over time, leading to a restart of the trial a couple years later. However, this start-and-stop of trial led to the Portico being compared to several iterations of the commercially available comparator devices, which are now on their third or fourth iterations, which improved their performance over the course of the trial.
Some TAVR experts at TCT, including Martin Leon, M.D., director of the Center for Interventional Vascular Therapy at NewYork-Presbyterian/ Columbia University Medical Center, questioned the results since it includes comparison data from older iterations of the devices that are no longer used. He and others said the bar needs to be set higher since the current generation of TAVR technology has improved significantly since 2014, when the Portico Trial first began.
Leon's comments were made during the FDA town hall meeting sessions focused on what will be needed for future clinical trials of new transcatheter valve technologies or FDA indications. The day closed out with a discussion transcatheter aortic valve replacement (TAVR) for patients with bicuspid leaflet valves.
The current indication for FDA cleared TAVR devices allows for use in these patients. However, there is not a lot of data on this population. Expert presentations explained the main issue is how much calcium is on the valve and where it is located, because some patterns of stenosis appear fine to treat with TAVR, but other types can block flow to the coronary arteries. Currently there is no standard for bicuspid patient selection for TAVR.
Leon said the possibility of a large, randomized controlled trial for how to treat bicuspid valves is remote, since the FDA already allows bicuspid TAVR. Panelists said data could be gathered using the TVT Registry, but it would not be ideal.
Read about a new option discussed at the town hall to pretreat bicuspid valves to safely allow TAVR use - BASILICA Procedure Prevents Coronary Obstruction From TAVR.
Watch the VIDEO: SCAI Prospective on Key Takeaways at TCT 2019 — Interview with Chandan Devireddy, M.D.
TAVR Leaflet Thrombosis, Thickening is Benign
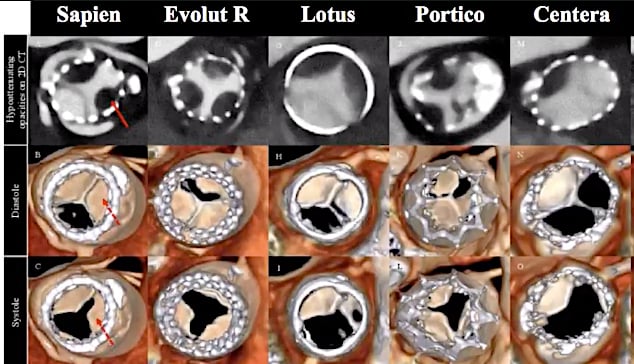 When hypo-attenuated leaflet thickening (HALT) was first discovered on CT scans in the Portico trial a few years ago, it caused great alarm that it could jeopardize the future of TAVR. However, subsequent studies found HALT occurs on all valves, TAVR and surgical, but the question remained on what to do about it.
When hypo-attenuated leaflet thickening (HALT) was first discovered on CT scans in the Portico trial a few years ago, it caused great alarm that it could jeopardize the future of TAVR. However, subsequent studies found HALT occurs on all valves, TAVR and surgical, but the question remained on what to do about it.
New data presented from the PARTNER 3 Low-Risk Computed Tomography Substudy: Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves, showed monitoring patients over a year found the thrombus formation is benign. It disappears over time without any intervention, such as anticoagulation. ALT and abnormal motion were more evident at 30 days among patients with severe aortic stenosis treated with TAVR in the low-risk PARTNER 3 trial, but by one year, the rates did not differ between surgical and transcatheter treatment arms. Both HALT and reduced leaflet motion resulted in slightly higher mean aortic valve gradients at 30 days and one year, but it was considered clinically insignificant.
"When this was originally presented, a lot of us were very concerned that there was a higher rate of this and maybe this was a sign of a burgeoning epidemic under the surface, but given this data, I think we feel relieved that we have been doing the right thing," Devireddy explained. He said it is still good to be vigilant and identify patients who are at a high risk for clotting for anticoagulation prophylaxis.
New COAPT Trial Data on MitraClip
 The COAPT Trial, first presented at TCT 2018, showed use of the Abbott MitralClip in heart failure patients with secondary mitral regurgitation (MR) can greatly improve patient symptoms and was superior to guideline-directed medical therapy alone. It changed the way cardiologists looked at secondary MR in heart failure and the FDA looked at the data and cleared a new indication for MitraClip in 2019 to treat these patients. Additional COAPT data has been presented as late-breakers at ACC.19 and TCT 2019.
The COAPT Trial, first presented at TCT 2018, showed use of the Abbott MitralClip in heart failure patients with secondary mitral regurgitation (MR) can greatly improve patient symptoms and was superior to guideline-directed medical therapy alone. It changed the way cardiologists looked at secondary MR in heart failure and the FDA looked at the data and cleared a new indication for MitraClip in 2019 to treat these patients. Additional COAPT data has been presented as late-breakers at ACC.19 and TCT 2019.
The three-year results from the COAPT Trial presented at TCT 2019 showed continued improvements in prognosis in selected heart failure (HF) patients. In addition, those patients that crossed over and received the MitraClip after 24 months showed the same benefits as those who received the device at the beginning of the study.
“At 36 months, transcatheter mitral leaflet approximation with the MitraClip was safe, provided durable reduction in MR, reduced the rate of HF hospitalizations and improved survival compared to medical therapy alone,” said Michael J. Mack, M.D., medical director, cardiovascular surgery at Baylor Health Care System. “In addition, those patients who crossed over and received a MitraClip experienced fewer HF hospitalizations and deaths or HFHs within 12 months than those who did not crossover, with rates comparable to patients originally assigned to the MitraClip.”
A second COAPT late-breaker was a cost-effectiveness analysis, which found MitraClip was a cost-effective treatment strategy for patients with heart failure and severe secondary MR.
One measure of success of any interventional procedure is how many procedures an operator has performed. Another late-breaking study focused in the first analysis of MitraClip operator volume-outcome relationship in the United States, based on data from the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) TVT Registry. Improvements in patient outcomes flattened out after about 50 procedures, but there still were gains associated with increasing operator experience up to about 200 MitraClip implantations.
On the expo floor, Abbott showed the newest FDA-cleared version of the MitraClip, the G4. It allows each side of the clip to be engaged independently.
Related TCT 2019 News and Videos:
TCT 2019 Late-breaking Presentations
Image Gallery From the TCT 2019 Meeting
VIDEO: SCAI Prospective on Key Takeaways at TCT 2019 — Interview with Chandan Devireddy, M.D.
VIDEO: Cardiogenic Shock Initiative Continues to Reduce Mortality by 50 Percent — Interview with William O’Neill, M.D.
VIDEO: Overview of Short DAPT in High-risk Bleeding Patients Who Receive Stents — Interview with Robert Harrington, M.D.
VIDEO: The Expansion of TAVR Following the FDA Clearing its Use in All Patients — Interview with Torsten Vahl, M.D.
VIDEO: TWILIGHT Trial Shows Benefit to Ticagrelor Monotherapy After Stent Implantation — Interview with Roxana Mehran, M.D.
VIDEO: Highlighting Women Involved With Structural Heart Interventions — Interview with Vivian Ng, M.D.
VIDEO: Early Discontinuation of DAPT in High Bleeding Risk Patients With the Synergy Stent — Interview with Ajay Kirtane, M.D.
VIDEO: Justification for Hemodynamic Support in Complex PCI — Interview with Jeffrey J. Popma, M.D.
VIDEO: New PLATINUM Diversity Data Shows Early DAPT Cessation OK in Minorities With New Generation Stent — Interview with Roxana Mehran, M.D.,


 August 28, 2023
August 28, 2023 









