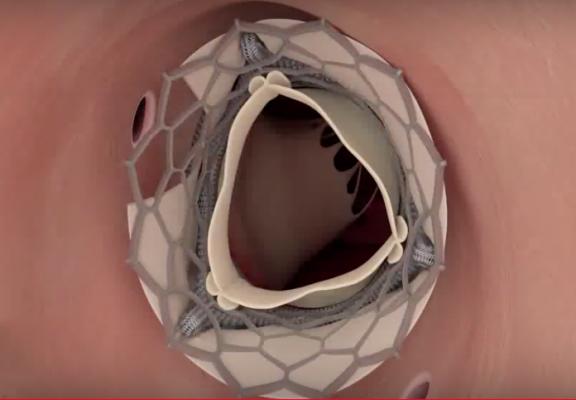
An illustration of the BASILICA procedure showing the leaflet of the original bioprosthetic valve sliced open to allow coronary blood flow after implantation of a TAVR valve. The new TAVR valve can push the leaflets of the bioprosthetic valve upwards, blocking the coronary arteries. During the BASILICA procedure, a catheter directs an electrified guidewire through the base of the left aortic cusp into a snare in the left ventricular outflow tract (LVOT). After snare retrieval, the mid-shaft of the guidewire is electrified to lacerate the leaflet, and the the leaflet splays after TAVR permitting coronary flow.
A novel technique has proven successful in preventing coronary artery obstruction during transcatheter aortic valve replacement (TAVR), a rare but often fatal complication. Called Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA), the technique will increase treatment options for high-risk patients who need heart valve procedures. The findings by researchers at the National Institutes of Health (NIH) were published in the Journal of the American College of Cardiology: Cardiovascular Interventions June 12, 2019.[1]
For elderly or frail patients, TAVR offers an effective and less invasive alternative to open heart surgery. However, a small subset of these patients may develop coronary artery obstruction during the TAVR procedure. For more than half of these patients, this complication has been fatal.
During TAVR, the surgeon places a catheter inside the heart and uses a balloon to open a new valve inside the aortic valve. However, in some patients whose hearts have uncommon structures, such as unusually large valve leaflets or small aortic roots, the large leaflets block the flow of blood to the coronary arteries as the new valve’s scaffolding opens.
BASILICA was developed at the National, Heart, Lung, and Blood Institute (NHLBI), part of NIH, to offer a solution to the problem of coronary obstruction during TAVR and increase the safety of TAVR for this subset of patients. The interventional cardiologist weaves an electrified wire the size of a sewing thread through a catheter and uses it to split the original leaflet in two so that it cannot block the coronary artery once it has been pushed aside by the new transcatheter heart valve. The procedure can be used on both the native valve leaflet or bioprosthetic valve leaflets in valve-in-valve (VIV) procedures.
Watch a VIDEO showing how the Basilica procedure is performed.
After animal experiments proved promising, the researchers successfully performed the procedure on seven patients who qualified for compassionate use of the technique — then untested in humans — because no other care options were available.
The current research builds on the success of the first-in-human trial.[2] From February to July 2018, the BASILICA technique was evaluated in a multicenter early feasibility study, sponsored by NHLBI. It enrolled 30 gravely ill patients who were at high or extreme risk if undergoing surgery. According to the researchers, all patients survived the procedure and underwent a successful TAVR. BASILICA was successful in 93 percent of patients and was feasible in natural as well as prosthetic aortic valves. At the 30 days mark, there were no coronary artery obstructions, nor a need to repeat the procedure due to valve dysfunction.
Every year, about 5 million people in the United States are diagnosed with heart valve disease, and more than 20,000 die, according to the American Heart Association (AHA).
Robert J. Lederman, M.D., senior investigator, NIH, NHLBI Division of Intramural Research, and Jaffar M. Khan, M.D., staff clinician, NIH, NHLBI led the study. Other researchers also contributed substantially to this project, including Adam B. Greenbaum, M.D., and Vasilis C. Babaliaros, M.D., from Structural Heart and Valve Center, Emory University Hospital; and Toby Rogers, BM BCh, Ph.D., from Medstar Washington Hospital Center.
This procedure is similar to the LAMPOON technique to reduce LVOT obstruction in transcatheter mitral valve replacement (TMVR).




 November 14, 2025
November 14, 2025 









