
Figure 1: Illustration showing the catheter steps for the LAMPOON procedure. From Dr. Jaffar M. Khan and the Laboratory of Cardiovascular Intervention, led by Dr. Robert J. Lederman.
Researchers at the National, Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH), developed a novel technique that prevents the obstruction of blood flow in the left ventricular outflow tract (LVOT) during transcatheter mitral valve replacement (TMVR). LVOT obstruction is a common fatal complication of TMVR, due to the close proximity off the aortic and mitral valves and the potential overhang of transcatheter valve blocking flow through the LVOT.
The new method called LAMPOON might increase treatment options for high-risk surgical patients who previously were ineligible for heart valve procedures. The Journal of the American College of Cardiology published the findings online May 20.[1] See Figure 1 for an illustration of the procedure.
TMVR is used to treat mitral valve stenosis and regurgitation. Untreated, these conditions can cause pulmonary hypertension, heart enlargement, atrial fibrillation, blood clots, and heart failure.
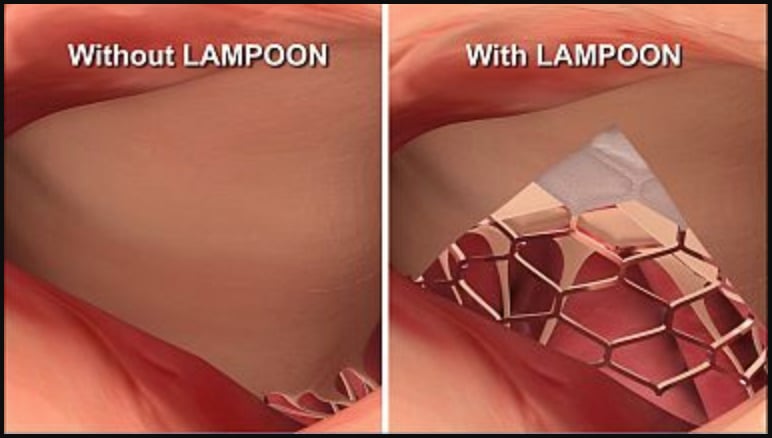 For elderly or frail patients, TMVR offers a less-invasive alternative to open heart surgery. During TMVR, doctors replace the mitral valve by delivering an artificial valve through a catheter. But in more than 50 percent of patients, the heart’s anatomy gets in the way. The heart leaflet is pushed back and blocks blood flow, causing LVOT obstruction. See Figure 2 for an illustration of LVOT obstruction.
For elderly or frail patients, TMVR offers a less-invasive alternative to open heart surgery. During TMVR, doctors replace the mitral valve by delivering an artificial valve through a catheter. But in more than 50 percent of patients, the heart’s anatomy gets in the way. The heart leaflet is pushed back and blocks blood flow, causing LVOT obstruction. See Figure 2 for an illustration of LVOT obstruction.
“These patients have a failing mitral valve, are not able to undergo open heart surgery, and are now rejected as candidates for TMVR because of the very high risk of left ventricular outflow tract obstruction,” said study author Jaffar M. Khan, M.D., clinician at NHLBI.
To increase the availability of TMVR for this subset of patients, Khan and colleagues at NHLBI and Emory University developed a procedure that makes an intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction, dubbed LAMPOON.
Overview of the LAMPOON Procedure
In the LAMPOON procedure, the operator inserts two catheters through the patient’s groin, and then through the blood vessels until it reaches the heart. The doctor then uses an electrified wire the size of a sewing thread woven through the catheter to split open the leaflet. At that point, the patient is ready to undergo TMVR.
“Surgeons cut out the leaflets when they replace valves. They can do it, because they have cut open the chest and the heart and can clearly see the problem. LAMPOON is designed for patients who need a new mitral valve, but can’t, or may not want to undergo open heart surgery,” said Khan.
According to the researchers, other preventive strategies have had largely suboptimal outcomes.
LAMPOON Study Data Shows Increased Survival
Between June 2017 and June 2018, the LAMPOON Study enrolled 30 patients, median age 76, considered at high risk for surgical valve replacement and at prohibitive risk of LVOT obstruction during TMVR.[2]
All patients survived the procedure and 93 percent reached the 30-day survival mark, which compares favorably to a 38 percent reported with other methods. The primary outcome of the study, which combined a successful LAMPOON, followed by a successful TMVR without re-intervention, was achieved in 73 percent of the patients.
The researchers hope the technique will eventually help reduce the number of deaths from heart valve disease. Every year, approximately 5 million people in the United States are diagnosed with heart valve disease, and more than 20,000 Americans die of the disease each year, according to the American Heart Association (AHA).
Read the related article "Henry Ford Hospital Cardiologist Performs Pioneering Laser Mitral Valve Treatment," from the LAMPOON Study.
About the National Heart, Lung, and Blood Institute (NHLBI) is part of the National Institutes of Health (NIH). NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information: www.nhlbi.nih.gov.
Related Mitral LVOT Obstruction Content:
VIDEO: The Importance of the Neo-LVOT in Transcatheter Mitral Valve Replacement — Interview with Dee Dee Wang, M.D.,
The Essentials of Structural Heart Imaging
VIDEO: Evaluation Tool for Neo-LVOT in Transcatheter Mitral Valve Procedures



 January 05, 2026
January 05, 2026 









