October 10, 2019 — An independent analysis of Lutonix 035 drug-coated balloon (DCB) patient-level data showed no statistically significant mortality increase. This was one of the first major reviews of trial data for patients who were treated with a paclitaxel coated device used in peripheral vessels following a meta analysis study of paclitaxel device trials published in December 2018 that showed a mortality safety signal. This was a major discussion during the FDA townhall meeting at the Transcatheter Cardiovascular Therapeutics (TCT) 2019 meeting, where this late-breaking trial was presented.
The study was published in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions and simultaneously presented in a late-breaking session at TCT.
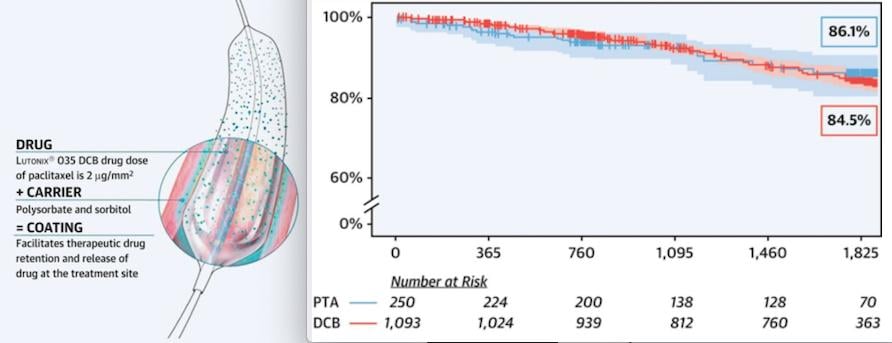
This independent analysis evaluated Lutonix 035 DCB (N=1093) and standard percutaneous transluminal angioplasty (PTA) (N=250) safety outcomes using patient-level data and propensity-matching from three Lutonix DCB randomized, controlled trials: LEVANT 1, LEVANT 2 and LEVANT Japan, as well as the Continued Access cohort of the LEVANT 2 trial, and confirmed that there was no statistically significant increase in mortality with the use of Lutonix 035 DCB.
“While the FDA and Advisory Committee of Circulatory System Devices Panel in June identified a late mortality signal after treatment with paclitaxel-coated devices using a meta-analysis of randomized, controlled trials at five years from multiple companies, they recognized the benefits of these devices, including fewer reinterventions. They agreed that the magnitude of the signal should be interpreted with caution due to multiple limitations in the available data,” said Kenneth Ouriel, M.D., president and CEO, Syntactx, the clinical research firm that conducted the independent analysis, and former chairman of Surgery at the Cleveland Clinic. “This large patient-level analysis found no statistically significant increase in mortality associated with Lutonix DCB treatment. Lutonix DCB remains a viable medical therapy for patients with peripheral arterial disease who demonstrate a high risk for restenosis and repeat femoropopliteal interventions.”
Further, a medical advisory committee comprised of an interventionalist and oncologist re-evaluated the cause of death in the LEVANT 1 and LEVANT 2 trials, including the LEVANT 2 Continued Access cohort. This independent review confirmed through adjudication that zero deaths were determined to be related to paclitaxel. Moreover, no clustering or pattern of death in any cardiovascular or non-cardiovascular categories was observed, which would have indicated a causal relationship between paclitaxel and death.
“The published patient-level analysis from Syntactx provides important information that health care providers can use to make an informed decision on the use of paclitaxel devices until additional long-term data are available,” said J.D. Meler, M.D., vice president of Medical and Clinical Affairs for BD’s Peripheral Intervention business. “Patient safety is our top priority, and BD remains committed to improving the quality of life of patients with PAD.”
No Increased Long-Term Mortality for the Paclitaxel-Coated Zilver PTX Stent (November 2019 update)
6 Hot Topics in Interventional Cardiology at TCT 2019
Find more late-breaking news and video from TCT 2019
Reference:


 June 13, 2024
June 13, 2024 









