Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash and stock transaction.i "Penumbra is a ...
Venous Therapies
This channel includes news and new technology innovations of Venous Therapies including deep vein thrombosis (DVT) and vena cava filters.
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted ...
Sept. 9, 2025 — The American Venous Forum (AVF), International Society on Thrombosis and Haemostasis (ISTH), National ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
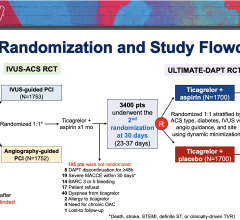
April 8, 2024 — People who have had a heart attack or who are at risk for a heart attack and who stopped taking aspirin ...
March 21, 2024 — Prolocor, Inc., a healthcare startup developing an innovative precision diagnostic test with the goal ...
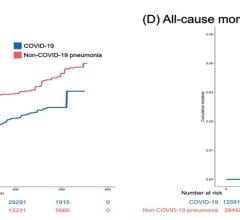
January 11, 2024 — Acute cardiovascular manifestations of COVID-19, such as heart failure, thrombosis, and dysrhythmia ...
October 31, 2023 — Results of four late-breaking clinical trials presented during the The VEINS Conference 2023, taking ...
January 11, 2023 — The Alameda, Calif.-based global healthcare company Penumbra has announced U.S. Food & Drug ...
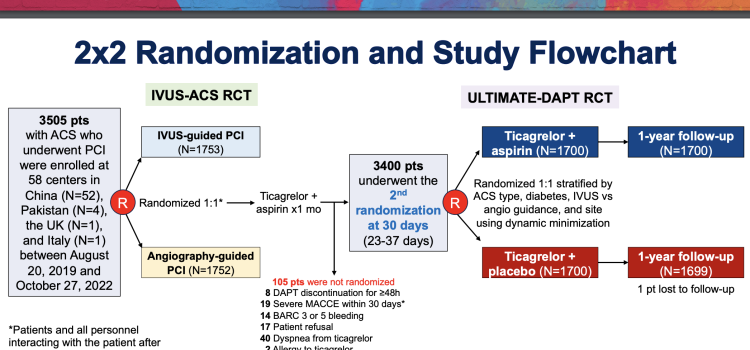
November 21, 2022 — New research published in the Journal of the American College of Cardiology shows the use of drug ...
November 2, 2022 — The second round of late-breaking clinical trial results were announced at VIVA22 on Nov. 1 in Las ...
November 2, 2022 — A number of awards of distinction were presented during The VEINS (Venous Endovascular INterventional ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...
The global thrombectomy devices market is poised to experience substantial expansion, owing to the emergence of ...


 January 19, 2026
January 19, 2026