Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash and stock transaction.i "Penumbra is a ...
Deep Vein Thrombosis (DVT)
Deep vein thrombosis (DVT) is caused by the formation of venous blood clots in the arms and legs. This page includes information on technologies to treat DVT using catheter based interventions and thrombolysis.
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
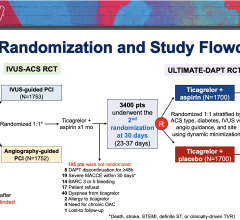
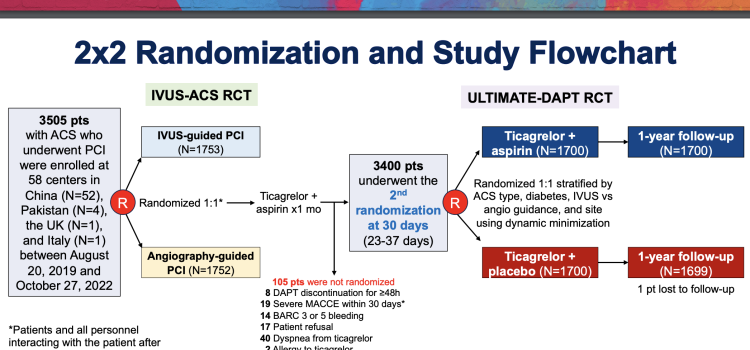
April 8, 2024 — People who have had a heart attack or who are at risk for a heart attack and who stopped taking aspirin ...
March 21, 2024 — Prolocor, Inc., a healthcare startup developing an innovative precision diagnostic test with the goal ...
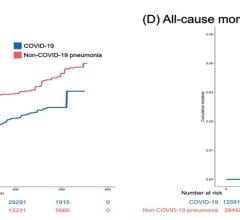
January 11, 2024 — Acute cardiovascular manifestations of COVID-19, such as heart failure, thrombosis, and dysrhythmia ...
October 31, 2023 — Results of four late-breaking clinical trials presented during the The VEINS Conference 2023, taking ...
November 21, 2022 — New research published in the Journal of the American College of Cardiology shows the use of drug ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...
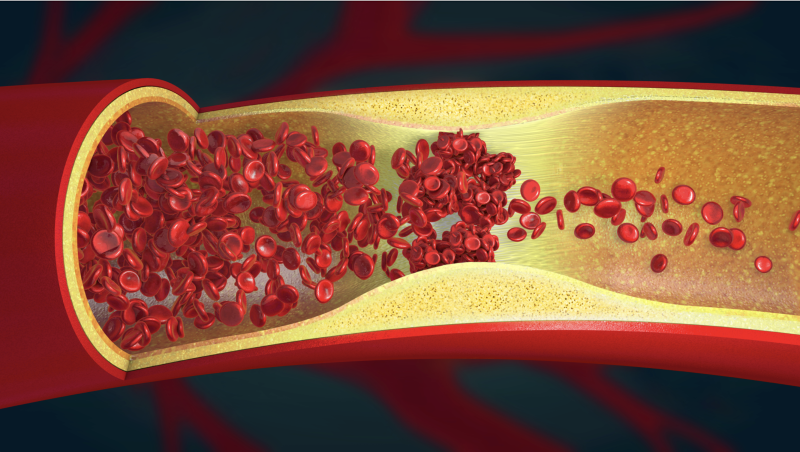
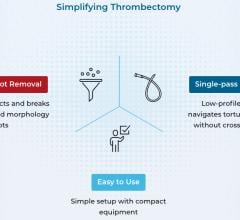
The global thrombectomy devices market is poised to experience substantial expansion, owing to the emergence of ...
May 20, 2022 — Results from a real-world study investigating safety and effectiveness of clopidogrel versus aspirin ...
January 10, 2022 – Akura Medical Inc., a Shifamed portfolio company, announced the closing of its $25 million Series A1 ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
Behnood Bikdeli M.D., a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, offers an ...
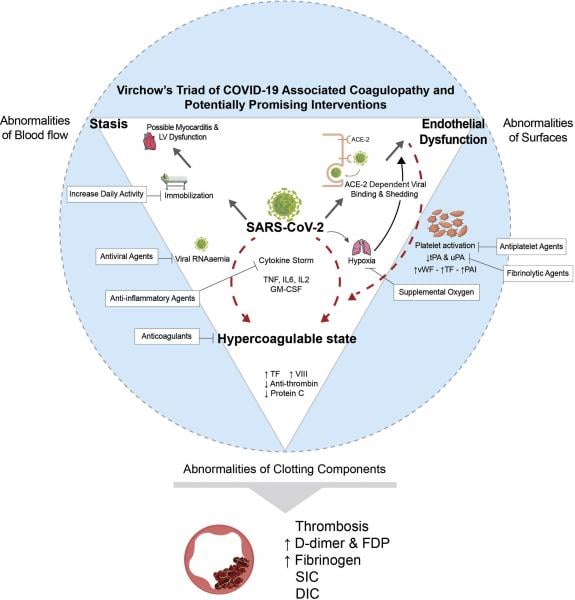
A comprehensive review or more than 80 randomized controlled trials (RCTs) investigating how to best manage optimal ...
January 8, 2021 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Inari Medical Inc ...




 January 19, 2026
January 19, 2026