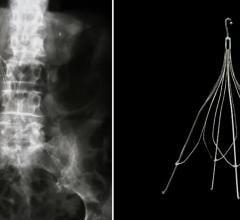
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has authorized marketing of the first laser-based device for the removal of inferior vena cava (IVC) filters. The device is designed for ...
Vena Cava Filters
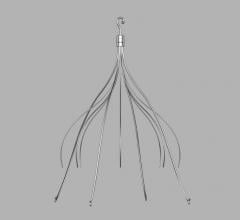
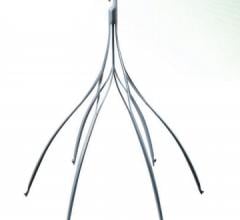
Vena cava filters are a type of venous therapy that involves implanting a self-expanding wire cage medical device into the inferior vena vein to prevent pulmonary emboli traveling from the lower extremities into the lungs to cause a pulmonary emolism (PE).
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has authorized marketing of the first laser-based device ...
July 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation (BDD) to a laser ...
September 9, 2020 — The Society of Interventional Radiology (SIR) published new clinical practice guidelines that ...
December 4, 2018 — BTG plc announced the first patients outside of a clinical trial have been successfully implanted ...
January 2, 2018 — Osborne & Associates Law Firm, P.A., of Boca Raton, Fla., has filed suit in Palm Beach Circuit Court ...
September 26, 2017 — The one-year results of the SENTRY clinical trial were presented by principal investigator Michael ...

September 14, 2017 — Here are quick summaries for all the key late-breaking vascular and endovascular clinical trials ...
January 21, 2015 — When retrievable inferior vena cava (IVC) filters were approved for use in the United States in 2003 ...

January 5, 2015 — The first large-scale, multispecialty prospective clinical research trial to evaluate the use of infer ...
August 6, 2014 — Medical market research group Decision Resources finds that the United States clot management device ...
June 5, 2014 — Cook Medical is engaged in two clinical studies that will provide additional data on the safety and ...
April 18, 2013 — One venous puncture, rather than two, is a safe and effective approach to intravascular ultrasound-guid ...
April 5, 2013 — Continuing its commitment to provide best-in-class medical devices for the prevention of recurrent ...
This video, provided by Crux Biomedical, demonstrates the implantation of the FDA-cleared Crux VCF inferior vena cava ...

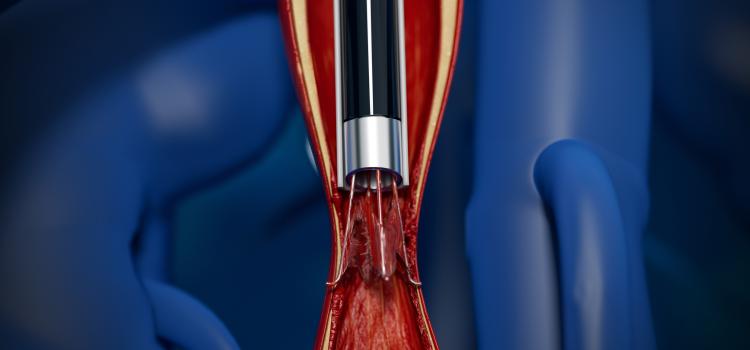




 January 04, 2022
January 04, 2022