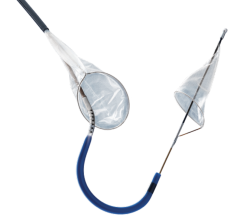
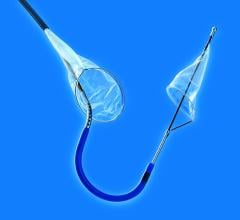
April 5, 2013 — Continuing its commitment to provide best-in-class medical devices for the prevention of recurrent pulmonary embolism (PE), Argon Medical Devices Inc. launched the OptionELITE Retrievable Vena Cava Filter. The OptionELITE IVC Filter, designed by Rex Medical LP, provides the clinician with the same safe and trusted design now enhanced for ease of delivery and retrievability.
"Safety and retrievability are without question two of the most vital, and rare, characteristics in a best-in-class retrievable IVC filter. Argon is pleased to be able to offer the best of these worlds by introducing the OptionELITE," stated George Leondis, president of Argon Medical Devices. "This technology provides clinicians with a safe and stable IVC filter that can be confidently deployed and retrieved while minimizing the risk of migration, penetration and fracture. This product, along with the new Cleaner15 Mechanical Thrombectomy System, and the recently announced acquisition of Angiotech's interventional business, positively positions the company as a significant player in the interventional arena."
OptionELITE's enhanced retention anchor pattern provides stability and reduced retrieval force while preventing migration and reducing the risk of penetration. The sturdy apex design has an increased capture zone for ease of snaring. In addition, the new high performance spiral sheath remains the lowest profile IVC filter delivery system available today (6.5 French OD, 5 French ID) and has been reinforced for improved kink-resistance and pushability.
"I have used Option for many years. At Weill Cornell, we had the opportunity to participate in the OptionELITE evaluation and found the device to be just as safe and stable as the Option, with no migrations and significantly easier to retrieve," said David W. Trost, M.D., associate professor of clinical radiology, Weill Medical College of Cornell University in New York, N.Y.
Argon Medical will be featuring the OptionELITE IVC Filter at the 38th Annual Scientific Meeting of the Society of Interventional Radiology in New Orleans, La., April 13-18, 2013 and will be offering a hands-on simulator training experience at booth 840.
PE affects an estimated 600,000 to 1 million people in the United States and its incidence is increasing annually. PE is the sudden blockage of an artery in the lung (pulmonary artery) typically thrombus. Emboli dislodgement can be caused by peripheral vascular disease (PVD), severe deep vein thrombosis (DVT), trauma and prolonged immobilization often following major surgical procedures. PE occurs when these clots break loose and embolize to block pulmonary blood vessels in the lungs. According to clinical research, if left untreated, PE has a mortality rate of 30 percent and is a leading cause of inpatient deaths in U.S. hospitals.
For more information: www.argonmedical.com


 April 25, 2023
April 25, 2023