January 11, 2023 — In recent years, transcatheter mitral valve replacement (TMVR) treatment and technology has evolved, but procedure-related morbidity remains a concern. A new study published in the ...
Embolic Protection Devices
This channel includes news and new technology innovations for embolic protection systems to prevent emboli from flowing down stream (distally) in arteries due to interventional or TAVR procedures.
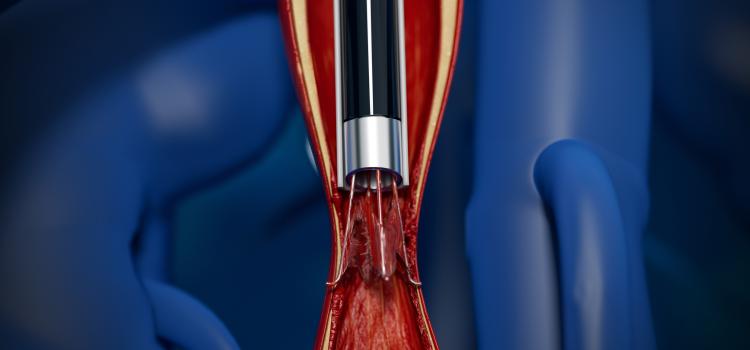
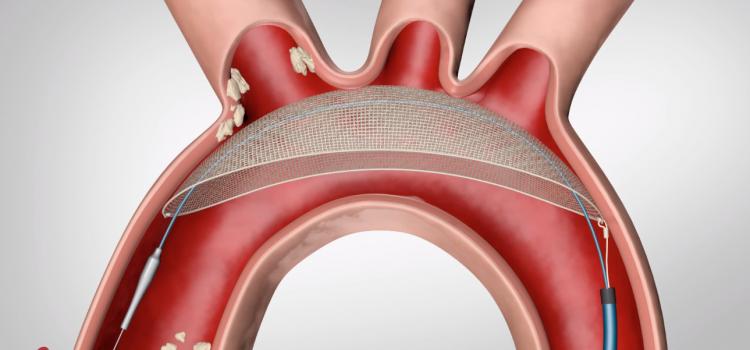
April 25, 2023 — FastWave Medical, a privately-held company founded by Big Sky Biomedical partners, announces the ...
January 19, 2023 — Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat ...
January 11, 2023 — In recent years, transcatheter mitral valve replacement (TMVR) treatment and technology has evolved ...
September 17, 2022 — Boston Scientific Corporation has announced results from the PROTECTED TAVR clinical trial ...
September 15, 2022 — Cardiac Implants LLC has announced the successful initial deployment of its Annuloplasty ring with ...
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has authorized marketing of the first laser-based device ...
February 9, 2021 — InspireMD Inc., developer of the CGuard Embolic Prevention System (EPS) for the prevention of stroke ...
October 15, 2020 – The REFLECT II randomized clinical trial evaluating the safety and efficacy of the Keystone Heart ...
September 9, 2020 — The Society of Interventional Radiology (SIR) published new clinical practice guidelines that ...
August 6, 2019 — Cardiovascular Systems Inc. (CSI) has acquired the Wirion Embolic Protection System and related assets ...
July 19, 2019 — Medical device startup company Filterlex Medical recently completed a series A financing round, raising ...
August 3, 2018 — Boston Scientific Corp. announced it has recently closed its acquisition of Claret Medical Inc., a ...
July 20, 2018 — Boston Scientific Corp. today has signed an agreement to acquire Claret Medical Inc., which has ...
June 7, 2018 — The first clinical cases have been completed where the Emboliner Embolic Protection Catheter was used ...
May 8, 2018 — One-year results from the Sentinel Cerebral Protection System show it can reduce the incidence of stroke d ...

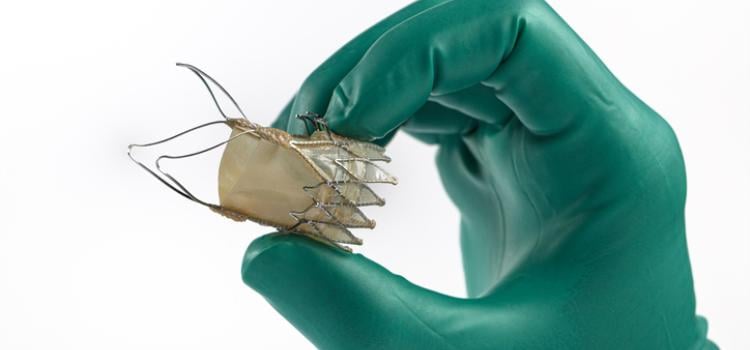
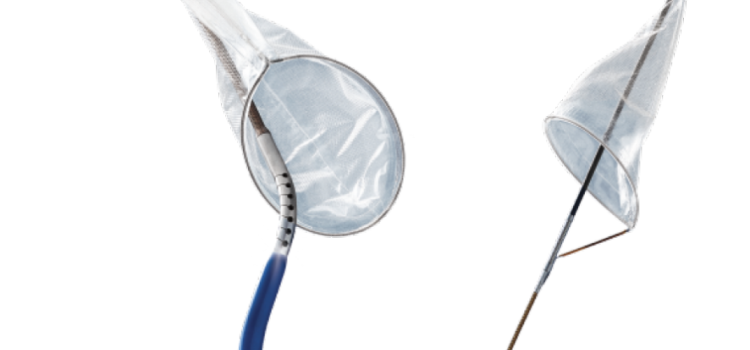

 April 25, 2023
April 25, 2023