Dean Kereiakes, MD, MSCAI
January 11, 2023 — In recent years, transcatheter mitral valve replacement (TMVR) treatment and technology has evolved, but procedure-related morbidity remains a concern. A new study published in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI) shows promise for intravascular lithotripsy (IVL)-assisted TMVR to treat a severely calcified mitral valve in a patient with severe stenosis and mitral regurgitation (MR).
MR affects an estimated 4 million people in the United States. Mitral valve leaflets become damaged or stretched and the valve may not close properly, which allows blood to flow backward, causing MR. Aortic stenosis, which is a narrowed aortic valve that decreases blood flow from the heart to the body, impacts as many as 300,000 people per year.
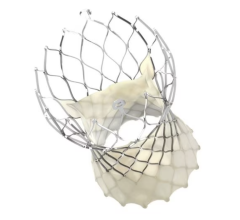
An 83-year-old male had previously received transcatheter aortic valve replacement (TAVR) for aortic stenosis presented with medically refractory dyspnea. He was enrolled in the APOLLO trial of Intrepid valve TMVR. Transseptal implantation of a 48-mm Intrepid valve was facilitated by Shockwave IVL delivered via two (8.0 x 60-mm) M5 + balloons placed across the mitral annulus before implantation. Cerebral embolic protection during IVL and valve implant was provided by Sentinel device and left subclavian balloon occlusion, according to the researchers.
“Our team used IVL to modify this severely calcified mitral annulus and valve leaflets to facilitate expansion of the Intrepid self-expanding valve,” stated Dean Kereiakes, MD, MSCAI, lead author of the study and Medical Director of The Christ Hospital Heart and Vascular Center, Medical Director of the Christ Hospital Research Institute, and Professor of Clinical Medicine at Ohio State University. “This type of patient is often turned down for catheter-based therapies.”
Post-dilation achieved valve frame expansion and reduced MR. Post-procedural imaging performed before hospital discharge and at 30 days displayed progressive valve frame expansion in the anteroposterior dimension, increased valve area, and resolution of MR.
Researchers stated that further clinical research and studies will be needed before IVL can be considered as a routine accompaniment to TMVR in patients with severe mitral annular and leaflet calcification or before Sentinel and balloon occlusion of the left subclavian can be proven to provide cerebral embolic protection during TMVR.
For more information: www.scai.org
Related content:
Shockwave Medical Initiates All-Female Coronary IVL Study
Transcatheter Cardiovascular Therapeutics (TCT) 2022 Conference Preview
Medtronic Intrepid Transcatheter Mitral Valve Successfully Uses Transfemoral Delivery


 May 05, 2025
May 05, 2025