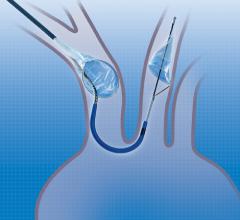
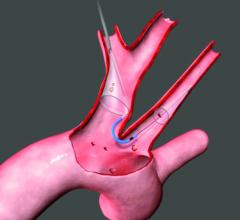
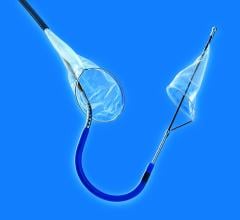
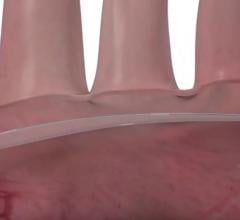
April 20, 2018 — Emerging medical device company Emboline Inc. announced it has completed a Series B funding round ...
Embolic Protection Devices
This channel includes news and new technology innovations for embolic protection systems to prevent emboli from flowing down stream (distally) in arteries due to interventional or TAVR procedures.
February 19, 2018 — Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017 ...
October 31, 2017 — The VAMPIRE 3 study evaluated if the selective use of distal filter protection might decrease the ...
October 23, 2017 — Claret Medical announced that it has closed on a Series C financing of $14.5 million led by ...
September 18, 2017 – Claret Medical announced publication of a new study in the Journal of the American College of ...
September 7, 2017 — Protembis GmbH announced the first clinical applications of its ProtEmbo Cerebral Protection System ...
August 23, 2017 — PinnacleHealth is the first hospital in Pennsylvania and one of the first 10 in the country to ...

June 5, 2017 – The U.S. Food and Drug Administration (FDA) granted market clearance for the Claret Medical Sentinel ...
May 17, 2017 — Contego Medical LLC announced recently that it has received CE Marking of its Vanguard IEP Peripheral ...
April 11, 2017 — Through a new clinical trial, patients at Harrisburg, Pa.-based PinnacleHealth have access to an ...
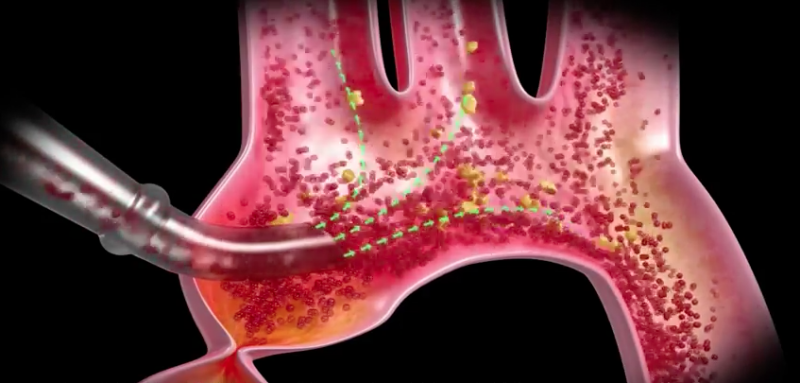
March 22, 2017 — Two U.S. Food and Drug Administration (FDA)-cleared embolic protection systems designed to remove ...
February 28, 2017 — A U.S. Food and Drug Administration (FDA) panel has recommended market clearance for the Claret ...
November 18, 2016 — InspireMD Inc. announced in mid-October the online publication of positive clinical data in a new ...
Studies have shown transcatheter aortic valve replacement (TAVR) has an increased risk of stroke and cerebral damage due ...
November 7, 2016 — Keystone Heart Ltd. announced that data presented at the Transcatheter Cardiovascular Therapeutics ...


 April 20, 2018
April 20, 2018