October 23, 2017 — Claret Medical announced that it has closed on a Series C financing of $14.5 million led by Lightstone Ventures, with participation from existing investors Easton Capital, HealthCor Partners, Incept LLC and Sante Ventures.
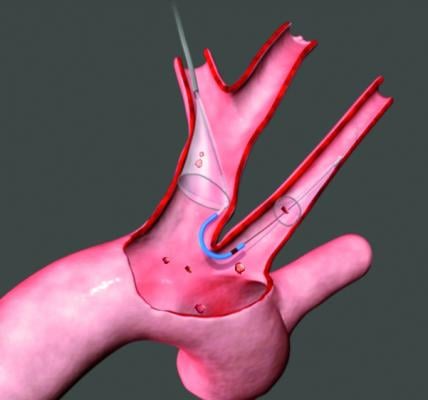
The company — developer of the Sentinel Cerebral Protection System, the first U.S. Food and Drug Administration (FDA)-cleared cerebral embolic protection device for transcatheter aortic valve replacement (TAVR) — will use the proceeds to support market access programs, research and development for next-generation products, and expansion of the commercial organization to build upon the successful controlled commercial release of the Sentinel Cerebral Protection System in the U.S.
Since the company’s Series B round in 2014, Claret Medical has pursued a structured study program of the Sentinel device involving three randomized studies and more than 2,300 patients, including its U.S. SENTINEL pivotal trial and a large-scale, real-world trial. It has also gained FDA clearance and successfully commercialized Sentinel in TAVR centers across Europe, selected Asia Pacific countries and, most recently, the U.S.
Sentinel is the first and only FDA-cleared device that captures and removes debris that is dislodged ubiquitously during TAVR, regardless of the TAVR system used or a patient’s risk profile, before it can travel to the brain and potentially cause neurological and neurocognitive damage.
For more information: www.claretmedical.com


 April 25, 2023
April 25, 2023 









