
July 20, 2018 — Boston Scientific Corp. today has signed an agreement to acquire Claret Medical Inc., which has commercialized an embolic protection filter to reduce the risk of strokes during transcatheter aortic valve replacement (TAVR) procedures. The purchase price was $220 million in up-front cash, as well as a potential reimbursement-based milestone payment of up to $50 million.
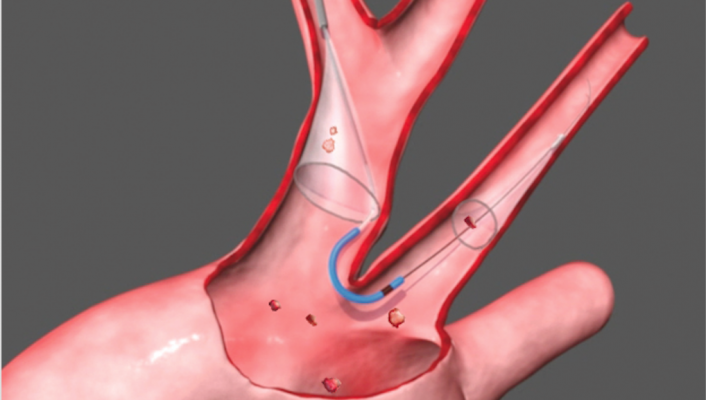
The privately held company developed and commercialized the Sentinel Cerebral Embolic Protection System. The device is used to protect the brain during certain interventional procedures, predominately in patients undergoing TAVR. The technology will expand Boston Scientific's structural heart portfolio, which includes the Watchman left atrial appendage ocular and the Lotus TAVR valve.
The Sentinel System received European CE mark in 2014 and U.S. Food and Drug Administration (FDA) clearance in 2017. It is the only device cleared to protect patients against the risk of stroke during TAVR, a minimally invasive procedure to replace the aortic valve in patients with severe aortic stenosis. Embolic debris such as calcium or tissue can embolism during the procedure, travel through the bloodstream toward the brain and potentially cause neurological and neurocognitive damage. Recent studies have estimated about 4 percent of patients experience a clinically apparent stroke within 30 days of a TAVR procedure.[1,2,3,4,5,6]
"Through the development and commercialization of the Sentinel System, Claret Medical has successfully introduced a new layer of safety and peace of mind for physicians and their patients undergoing TAVR procedures," said Kevin Ballinger, president, interventional cardiology, Boston Scientific. "This acquisition will expand our commercial portfolio to include an important adjunctive offering aimed at improving TAVR patient outcomes. We also see potential for future use in other left heart and endovascular procedures such as mitral valve repair and replacement, left atrial appendage closure and pulmonary vein isolation ablation procedures for atrial fibrillation."
Trial Evidence Supporting the Sentinel Device
In the pivotal SENTINEL Trial, the landmark study that led to FDA clearance and commercial introduction of this first-time therapy in the U.S., the Sentinel System reduced the incidence of strokes by 63 percent within the first 72 hours of the procedure.[7] In clinical studies, the Sentinel System captured debris flowing toward the brain in 99 percent of TAVR cases, regardless of the type of replacement valve that was used.[8]
"We have been pleased by the consistent, high-quality clinical evidence generated by the committed thought leaders at leading academic centers, as well as the rapid physician adoption of the device — utilized on average in 60 percent of TAVR procedures performed across 100 centers – within just 12 months of carefully controlled U.S. commercialization," said Azin Parhizgar, Ph.D., president and chief executive officer, Claret Medical. "Nearly 10,000 patients have been treated worldwide with the Sentinel System, and we are confident that the leadership of Boston Scientific will enable increased momentum and improved patient access to this valuable technology."
Claret Medical is based in Santa Rosa, Calif., and has about 120 employees. The acquisition is projected to close during the third quarter of 2018, subject to customary closing conditions.
The transaction is expected to be immaterial to adjusted earnings per share in 2018, accretive in 2019 and increasingly accretive thereafter. On a GAAP basis, the transaction is expected to be less accretive, or more dilutive as the case may be, due to amortization expense and acquisition-related net charges.
Related Content on the Sentinel Embolic Protection System
TAVR Embolic Protection Systems to Reduce Stroke Rates
Sentinel Cerebral Protection System Reduces TAVR Strokes More Than 60 Percent
Sentinel Cerebral Protection System Reaches 50-Center Adoption Milestone in U.S.
Sentinel Cerebral Protection System Significantly Reduces Stroke and Mortality in TAVR
PinnacleHealth First in Pennsylvania to Implant Sentinel Cerebral Protection System
FDA Clears First TAVR Embolic Protection System
TCT 2014: Editor's Choice of the Most Innovative New Technologies
References:
7. Samir Kapadia S, et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. JACC. Jan 2017; 69(4): 367-377.
8. Tobias Schmidt T, et al. Debris Heterogeneity Across Different Valve Types Captured by a Cerebral Protection System During Transcatheter Aortic Valve Replacement. JACC: Cardiovascular Interventions. Jul 2018; 11(13): 1262-1273.


 November 14, 2025
November 14, 2025 









