While transcatheter aortic valve replacement (TAVR) is a paradigm shift in how valve disease is treated, one nagging safety issue that remains is TAVR’s stroke rate, which is higher than traditional surgical valve replacements. The solution to this issue may be the use of specialized embolic protection systems, of which four companies displayed their devices on the show floor and in presentations at the 26th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in September.
Data was presented from the CLEAN-TAVI trial, which is a first-of-its kind study using a cerebral protection device during TAVR. Data showed the use of embolic protection significantly reduced the number and volume of cerebral lesions in high-risk patients with severe aortic stenosis.
“Despite improvement in case selection, TAV devices, procedural; techniques and adjunctive pharmacology, embolic strokes are still a devastating complication after TAVR,” said data presenter Martin Leon, M.D., director of the Center for Interventional Vascular Therapy, Columbia University Medical Center / New York-Presbyterian Hospital. "If additional studies confirm consistent reductions in neuro-imaging stroke lesions, especially if correlated with improvement in clinical neurological endpoints, then cerebral protection with TAVR will becaome the standard of care in the future."
TAVR Stroke Rates
The valves being replaced in TAVR procedures are usually heavily calcified and require balloon valvuloplasty prior to the placement of the device. Placement using the Edwards Lifesciences Sapien valve requires another balloon inflation to expand the stent support structure of the valve. Medtronic’s Corevalve is self-expanding and is deployed by pushing it out of a catheter into position. Both the valvuplasty and valve deployment are blamed for releasing emboli, which can flow directly up the carotid arteries to the brain, causing strokes.
Stroke rates for TAVR in trials for the Corevalve range from 2 to 5.8 percent.[1, 4] In trials for Edwards Lifesciences Sapien valve, the stroke rate ranges from 5 to 7.8 percent.[2] Data collected the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry from 7,710 patients who underwent TAVR showed stroke rate about 2 percent.[3] Surgical valve replacement stroke rates are between 1.1 and 7 percent.[2, 4]
The CoreValve U.S. Pivotal Trial High Risk Study paid closer attention to stroke and detected more of them than in previous trials. Post procedural stroke rates of 3.9 for Corevalve and 3.1 for surgical repair were reported, which rose to 5.8 and 7 percent by one year.[4] While higher than reported rates in earlier trials, the stroke percentages were statistically comparable between surgical and TAVR arms of the trial, said Co-Principal Investigator David Adams, M.D., professor and chair, cardiothoracic surgery, Mt. Sinai Hospital, New York. He said the stoke rate might be improved with the use of embolic protection systems in development.
TAVR Embolic Protection Systems
Two companies at TCT 2014, Claret Medical and Keystone Heart, displayed their devices on the show floor. Claret’s Sentinel Cerebral Protection System also was the subject of a clinical trial presentation, in which its devices was used in a first-in-human study that showed a significant reduction in stroke. Two other systems were discussed in sessions.
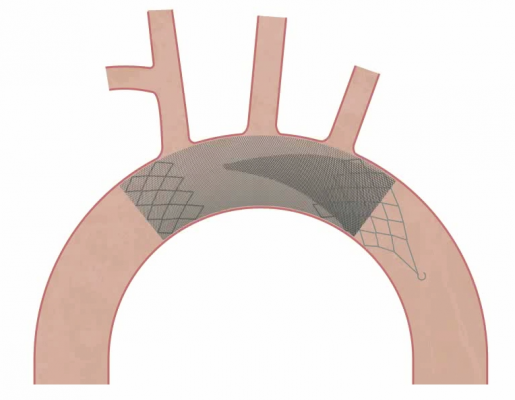
Claret’s Sentinel system is composed of a double embolic filter net, one for each carotid artery. The system combines a guidewire, support catheter and the two embolic nets into one catheter. Using a 6 French radial artery access point, the catheter is passed through the brachiocephalic artery into the aortic arch. There the tip is manipulated to make an extreme bend into the neighboring left common carotid artery and the first net is deployed just past the ostium inside the vessel. The second net is then deployed inside the brachiocephalic artery.
Claret started its pivotal SENTINEL Trial, its U.S. Food and Drug Administration (FDA) study to seek FDA approval. The first patient was treated in the trial on October and the company expects to finish the trial by mid-2015. The company already has European approval for the device.
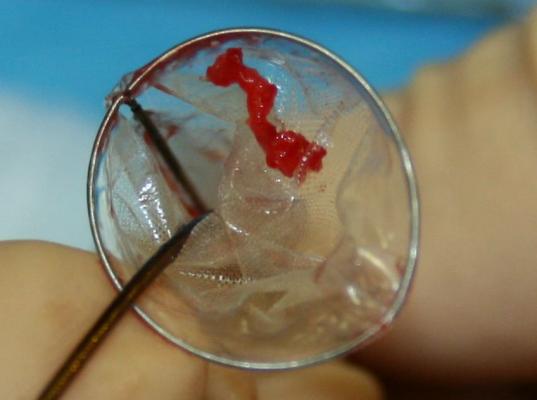
Keystone Heart’s Triguard device consists of a self-expanding nitinol frame covered in a mesh material to deflect emboli from traveling up any of the three vessels at the top of the aortic arch. It is deployed via a catheter introduced from the femoral artery. As the device is unsheathed, a nitinol stabilizing wire is expanded into the brachiocephalic artery to properly orientate the screen. A second stabilizing wire acts as a foot on the base of the device that touches the bottom of the aortic arch to help push the device upward to form a better seal against the top of the arch. After the procedure it is pulled back into the delivery catheter for removal. The net does not capture emboli, but does deflect it from the carotid vessels.
A third device discussed in sessions was the Edwards Lifesciences Embrella embolic deflector, which received Eurpean CE mark in 2010. The device is placed in the aorta through a sheath inserted in the right brachial or right radial artery. Its porous membrane allows blood flow to the brain while simultaneously deflecting embolic material. The device uses two self-expanding frames covered in a filter mesh, attached to a central catheter from which it is deployed and retrieved.
Data on the Embrella’s recent PROTAVI-C Pilot Study of 53 patients were presented at TCT. While the trial demonstrated the device is safe to deploy, it also showed its deployment appears to cause more ischemic defects in the brain than the control group receiving TAVR without embolic protection. All of these defects, evaluated via MRI, where small , clinically silent and resolved within a few weeks.
A fourth device, the Emboline Inc. Prosheath embolic protection system, was presented in a new technology session. It deploys a large self-expanding stent structure with a filter mesh covering along the length of the aortic arch into the decending aorta. At the base of the device where it is attached to its deployment catheter, it uses a reversed funnel shaped filter to capture all emboli released during the procedure. It is the first device proposed at TCT capable of capturing all emboli released during TAVR procedures. The company hopes to being first-in-human trials in 2015.
CLEAN-TAVI Trial Data
Data from the CLEAN-TAVI trial showed the use of Claret’s Sentinel device significantly reduced the number and volume of cerebral lesions in high-risk patients with severe aortic stenosis. It was a prospective, randomized, double-blind single-center study, and the first trial to examine the impact of a cerebral protection device in preventing magnetic resonance imaging (MRI)-detected brain lesions during TAVR. The primary endpoint was the number of lesions in the protected brain region as determined by diffusion-weighted MRI (DW-MRI) subtraction at two days post TAVR. Secondary endpoints included the total lesion volume at two and seven days after TAVR and lesion number at seven days.
The filter group at two days post-TAVR showed a significantly lower ataxia rates (24 vs. 9 percent) than the control group, which support the notion that the filter has the potential to improve neurological outcomes.
A total of 100 patients with severe aortic stenosis who were at increased surgical risk were recruited and randomly assigned in a 1:1 ratio to TAVR with cerebral protection (device group) or TAVR alone (control group). Patients underwent MRIs of the brain before and at two and seven days after TAVR. After two days, the median number of lesions in the protected regions in the device group was significantly lower than the control group (four versus 10, respectively, p=0.009). At seven days after TAVR, the median lesion number was also significantly lower in the device group compared to the control group (three versus seven, p=0.0023). In addition, the median total lesion volume in the protected area was significantly smaller in the device group compared to the control group at two days (246 mm3 versus 527 mm3, respectively, p=0.0023) and at seven days (101 mm3 versus 292 mm3, p=0.002).
“In patients with severe aortic stenosis who are at increased surgical risk, the use of a cerebral protection device during TAVR significantly reduced the number of cerebral lesions in the protected brain regions,” said lead investigator Axel Linke, M.D., a professor at the University of Leipzig Heart Center in Germany. “Device use also reduced the volume of cerebral lesions as determined by DW-MRI.”
References:
1. Popma JJ, et al. “Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery.” JACC 2014, doi: 10.1016/j.jacc.2014.02.556.
2. Martin Leon, Craig Smith, Michael Mack, et al. “Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery.” N Engl J Med 2010; 363:1597-1607October 21, 2010DOI: 10.1056/NEJMoa1008232
3. Michael Mack, Matthew Brennan, Ralph Brindis, et al. “Outcomes Following Transcatheter Aortic Valve Replacement in the United States.” JAMA. 2013;310(19):2069-2077. doi:10.1001/jama.2013.282043.
4. David Adams, Jeffrey Popma, Michael Reardon, et al. “Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis.” N Engl J Med 2014; 370:1790-1798May 8, 2014DOI: 10.1056/NEJMoa1400590





 April 25, 2023
April 25, 2023 









