April 20, 2018 — Emerging medical device company Emboline Inc. announced it has completed a Series B funding round totaling over $10 million for its total embolic protection device for transcatheter aortic valve replacement (TAVR) procedures. The funding includes $3 million in new equity financing from multiple investors led by SV Tech Ventures and Shangbay Capital, and over $7 million in conversion of previously-issued convertible notes.
"We have been closely monitoring the development of the Emboliner since 2015 and are encouraged by the potential this unique technology has to improve the safety of TAVR and other interventional procedures," said Peng Cheng, managing partner at SV Tech Ventures. "This is an exciting time for Emboline as they begin their clinical studies, and we look forward to continuing our work with the Emboline team to bring this technology to patients across the globe."
As part of the financing, ShangBay Capital's William Dai will join Cheng on the Emboline board of directors.
The working capital will be used to fund the SafePass Clinical trial for the Emboliner Embolic Protection Catheter, the subsequent European CE Mark submission and initial commercial launch.
"The risks of stroke and other neurological damage from TAVR have been getting increased attention in the cardiology community. We recognize that managing these risks will be critical to expanding TAVR into lower-risk patient populations," said William Dai, founding partner at ShangBay Capital.
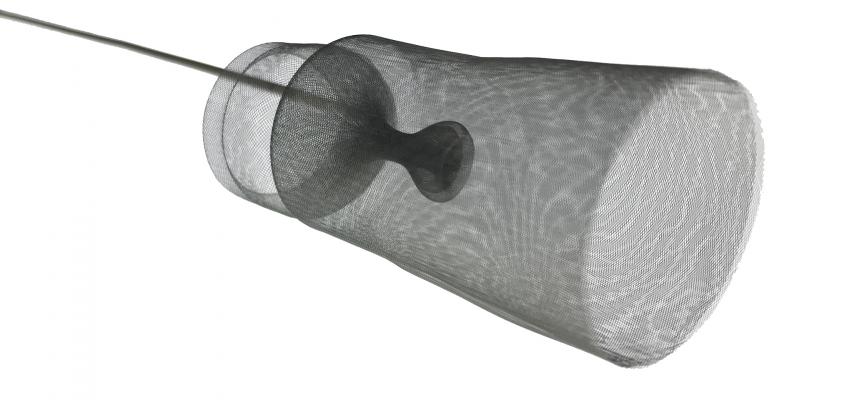
The Emboliner is a next-generation approach to reducing the risk of cerebral embolic events or stroke that patients may face following TAVR procedures. The device’s design presents significant improvements on first-generation solutions by offering the following:
- More effective coverage of all cerebral branches with fewer anatomical limitations;
- Full-body embolic protection, capturing both cerebral and non-cerebral debris;
- No additional procedural access required;
- Minimal interference with devices necessary to complete the procedure; and
- Outstanding ease-of-use and device control
For more information: www.emboline.com


 November 14, 2025
November 14, 2025 









