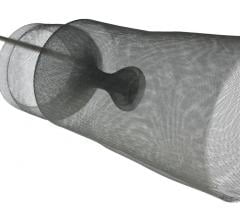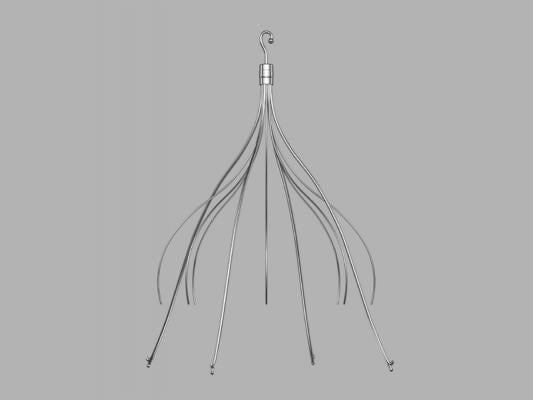
June 5, 2014 — Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava (IVC) filters.
The first study, the Cook Inferior Vena Cava Filter (CIVC) study, will add to Cook’s existing clinical data on its commercially available IVC filters. This includes three previous studies including more than 1,300 patients treated with Cook IVC filters. CIVC will also address filter safety concerns expressed in the 2010 and 2014 safety communications from the U.S. Food and Drug Administration (FDA). The study’s primary endpoints will be technical placement success and one-year freedom from new symptomatic pulmonary embolism, and one-year freedom from major adverse events.
CIVC will collect additional safety and effectiveness data on Cook’s permanent and retrievable filters. Enrollment in the CIVC study began in March 2014. Up to 470 patients at up to 40 international sites will be enrolled in the study.
The second study, the PRESERVE study, will involve collaboration between Cook, the Society for Vascular Surgery, the Society of Interventional Radiology, the FDA and other filter manufacturers, and will enroll patients in the United States only.
“Cook believes in providing physicians the data they need to make the best decisions possible when treating patients. In this case, these two studies should go a long way toward adding to the medical science on IVC filters,” said Mark Breedlove, vice president and global business unit leader for Cook Medical’s Peripheral Intervention clinical division.
For more information: www.cookmedical.com


 April 25, 2023
April 25, 2023