May 20, 2022 — Results from a real-world study investigating safety and effectiveness of clopidogrel versus aspirin ...
Venous Therapies
This channel includes news and new technology innovations of Venous Therapies including deep vein thrombosis (DVT) and vena cava filters.
Here are the top 10 performing videos on the Diagnostic and Interventional Cardiology (DAIC) website during the month of ...
January 10, 2022 – Akura Medical Inc., a Shifamed portfolio company, announced the closing of its $25 million Series A1 ...
January 4, 2022 — The U.S. Food and Drug Administration (FDA) has authorized marketing of the first laser-based device ...
November 22, 2021 — Fewer than 10 days of low-molecular-weight heparin (LMWH) after stenting for extensive iliofemoral ...
November 9 2021 — A new large-scale, real-world analysis of Centers for Medicare and Medicaid Services (CMS) outcomes ...
July 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation (BDD) to a laser ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
May 6, 2021 - Bluegrass Vascular Technologies announced the publication of a report documenting the clinical utility of ...
Behnood Bikdeli M.D., a cardiologist at the Brigham and Women’s Hospital, Harvard Medical School, Boston, offers an ...
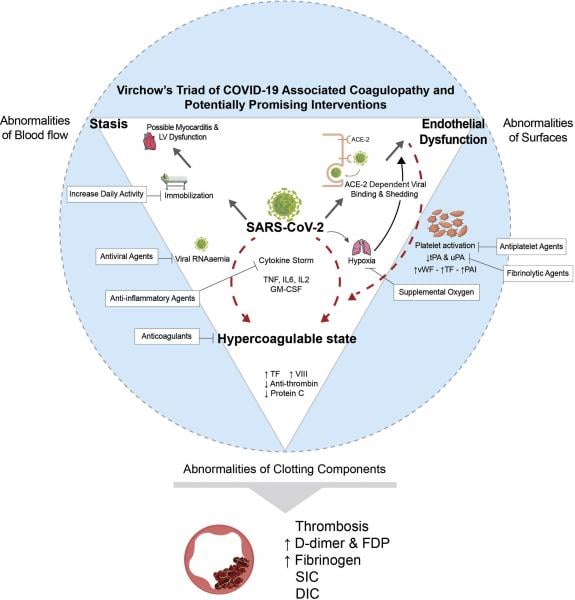
A comprehensive review or more than 80 randomized controlled trials (RCTs) investigating how to best manage optimal ...
February 22, 2021 — The U.S. Food and Drug Administration (FDA) recently cleared the Cook Medical Zilver Vena Venous ...
January 8, 2021 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Inari Medical Inc ...

December 23, 2020 — Three clinical trial platforms working together to test the effects of full doses of anticoagulants ...


 May 20, 2022
May 20, 2022