February 27, 2023 — Conformal Medical, Inc. announced today the results from the CONFORMAL Early Feasibility Study (EFS), evaluating the use of angiography compared to transesophageal echocardiogram (TEE) for left atrial appendage (LAA) assessment. The findings were presented at this year's Cardiovascular Research Technologies Conference (CRT 2023) in Washington, DC by Dr. William Gray, co-Director of the Lankenau Heart Institute and Professor of Medicine at Thomas Jefferson University during the Left Atrial Appendage Closure Forum.
"This study evaluated baseline TEE and angiographic data collected from 36 atrial fibrillation (AFib) patients who were deemed appropriate for left atrial appendage occlusion (LAAO)," stated Dr. Gray. "The complete paired images were analyzed for LAA diameter and depth to facilitate CLAAS device size selection. Results demonstrated a 97% agreement in device size selection between the two imaging modalities."
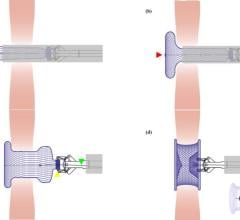
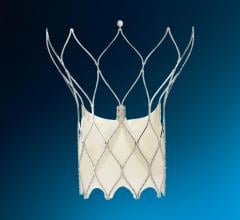
The CLAAS System is designed to seal the LAA in patients with non-valvular AFib to reduce the risk of stroke without the need for anticoagulants. Featuring a proprietary foam-based architecture, the implant addresses a wide spectrum of LAA anatomies with only two sizes. The system aims to simplify delivery and eliminate the need for procedural TEE so that physicians may perform the procedure without general anesthesia, a significant advancement with the potential to shift clinical practice to a same day, single operator procedure.
"The novel CLAAS device is designed to streamline LAAO procedures," commented Dr. Aaron Kaplan, professor of medicine at Dartmouth and co-founder and chief medical officer for Conformal Medical. "This study demonstrates that with only two sizes, the CLAAS implant requires minimal imaging, an important step as we look to transform the procedure to a single operator, same day procedure."
Conformal Medical is actively enrolling patients in the CONFORM pivotal trial, evaluating the safety and efficacy of the CLAAS System compared to other commercially available LAAO devices. The prospective, multicenter, randomized controlled study will enroll approximately 1,600 patients in the U.S. and will support U.S. Food and Drug Administration (FDA) pre-market approval.
For more information: https://conformalmedical.com/


 June 20, 2024
June 20, 2024