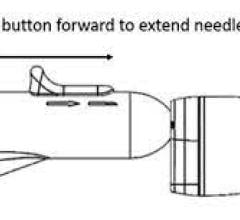
December 22, 2022 — Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac ...
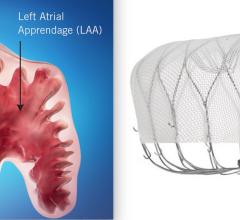
Left Atrial Appendage (LAA) Occluders
This channel includes news and new technology innovations about Left Atrial Appendage (LAA) Occluders. These close off the LAA in patients with atrial fibrillation to prevent the formation of stroke-causing clots in atrial fibrillation (AFib or AF) patients. LAA occlusion is often indicated for patients who do not tolerate anticoagulation therpy or have bleeding risks associated with use of that therapy.
Late-breaking clinical trials for the American College of Cardiology’s Annual Scientific Session Together with World ...
September 12, 2022 — Boston Scientific Corporation has received U.S. Food and Drug Administration (FDA) approval to ...
June 27, 2022 — Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac arrhythmias ...
May 25, 2022 — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food ...
April 12, 2022 – Patients who had leakage to the left atrial appendage due to incomplete device sealing after left ...
February 28, 2022 – Boston Scientific Corporation has announced positive results from a new analysis assessing real ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...
Christine Albert, M.D., MPH, professor, chair of the Department of Cardiology and the Lee and Harold Kapelovitz ...
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
October 6, 2021 — Boston Scientific Corp. announced it entered into a definitive agreement to acquire Baylis Medical ...
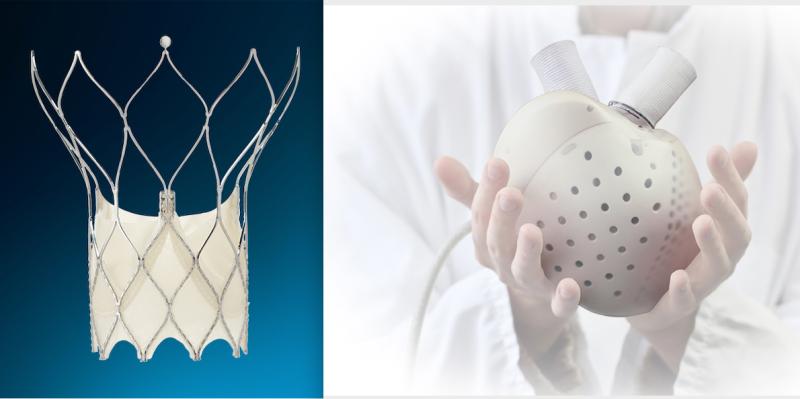
October 6, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
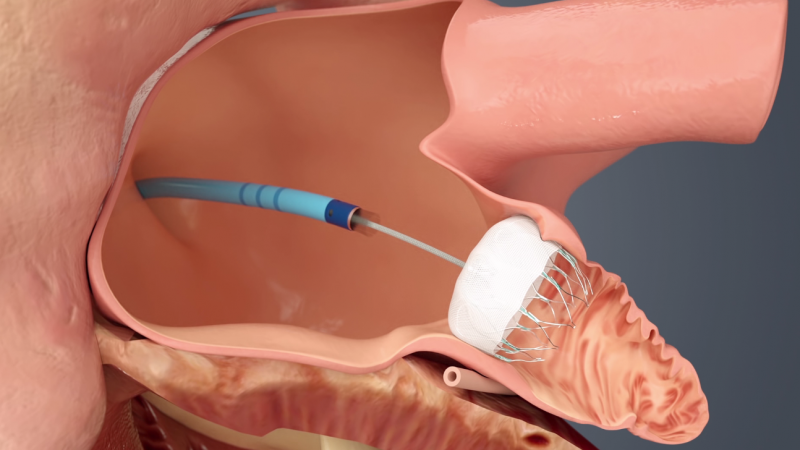
September 27, 2021 — Compared with men undergoing left atrial appendage occlusion (LAAO), women have a significantly ...
August 31, 2021 — Late-breaking data from a head-to-head clinical trial of the Amulet Left Atrial Appendage (LAA) ...

 December 22, 2022
December 22, 2022