December 22, 2022 — Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated, announced that the company has achieved the second milestone under the asset purchase agreement with Medtronic of its left-heart access portfolio with the submission for CE Mark of this portfolio under European Union (EU) Medical Device Regulations (MDR). This milestone triggers a $17 million earnout payment from Medtronic to Acutus.
“We are very pleased to have achieved this important milestone ahead of schedule. This submission is a critical step in our partnership with Medtronic, and we are confident that Medtronic will be able to accelerate adoption and positive impact on patient care and physician practice,” said David Roman, President & CEO of Acutus Medical. “In addition to positioning the left-heart access portfolio for long-term success in Europe, achieving this key milestone bolsters our financial position and enables Acutus to continue to focus on our differentiated mapping and therapy platform.”
Left-Heart Access Portfolio Sale
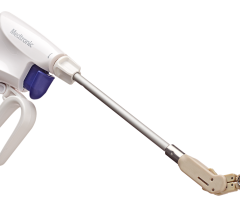
On June 30, 2022, Acutus completed the first closing of its previously announced sale of the Company’s left-heart access portfolio to Medtronic, which includes the AcQCross septal crossing devices, the AcQGuide MINI sheath and integrated crossing device, the AcQGuide FLEX Steerable Introducer with integrated crossing device, and the AcQGuide VUE steerable sheath.
Under the terms of the agreement, at the first closing, Medtronic paid cash consideration of $50 million to Acutus for, among other things, intellectual property rights to the Company’s left-heart access portfolio and certain equipment used in the manufacturing of these products. On October 31, 2022, Acutus completed the first major milestone in this transaction with OEM qualification and has now achieved the second major milestone of EU MDR filing. Starting January 28, 2023, Acutus will be eligible to receive four years of revenue-based earnouts.
For more information: https://www.acutusmedical.com/us/


 April 04, 2024
April 04, 2024