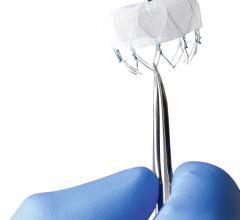
November 20, 2015 — Boston Scientific announced the first implants of the Watchman FLX left atrial appendage closure ...
Left Atrial Appendage (LAA) Occluders
This channel includes news and new technology innovations about Left Atrial Appendage (LAA) Occluders. These close off the LAA in patients with atrial fibrillation to prevent the formation of stroke-causing clots in atrial fibrillation (AFib or AF) patients. LAA occlusion is often indicated for patients who do not tolerate anticoagulation therpy or have bleeding risks associated with use of that therapy.
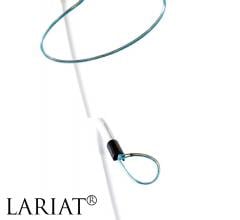
October 28, 2015 — SentreHeart Inc. announced that it has received CE Mark approval for the Lariat Surgical Left Atrial ...

July 13, 2015 - The U.S. Food and Drug Administration (FDA) is alerting healthcare providers and patients about reports ...
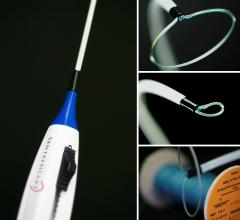
July 1, 2015 - SentreHeart Inc. announced that it has received approval for an Investigational Device Exemption (IDE) ...

June 30, 2015 - The American College of Cardiology (ACC), Heart Rhythm Society (HRS) and Society for Cardiovascular ...
Interview with Steve Goldstein, M.D., director, noninvasive lab, Medstar Washington Heart Institute, Washington, D.C ...
Role of Interventional Echcardiography in Transcatheter Structural Heart Procedures — Rebecca Hahn, M.D., Columbia ...
Interview with Ted Feldman, M.D., FACC, MSCAI, FESC, cardiac cath lab director, Evanston Hospital, North Shore Health ...
May 11, 2015 — Boston Scientific Corp. presented an overview of its continued business momentum and long-term growth ...
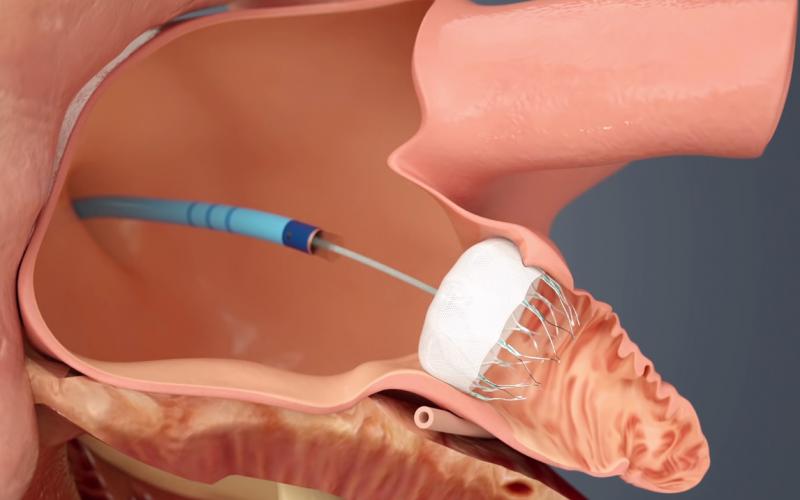
The long-awaited U.S. Food and Drug Administration (FDA) approval of the first transcatheter left atrial appendage (LAA) ...
March 25, 2015 — Mount Sinai experts were the first in the eastern United States on March 23 to implant the Watchman ...
DAIC Editor Dave Fornell shares his picks of the most interesting new devices and advances in cardiovascular technology ...
March 23, 2015 — AtriCure Inc. announced that it has launched the availability of its cryoICE cryo ablation probe (CRYO2 ...
March 13, 2015 — AtriCure Inc. announced the first two patients in the Dual Epicardial and Endocardial Procedure (DEEP) ...
As editor of DAIC, I keep a close watch on trends in cardiovascular technology and try to predict what the next big ...


 November 20, 2015
November 20, 2015