June 30, 2015 - The American College of Cardiology (ACC), Heart Rhythm Society (HRS) and Society for Cardiovascular Angiography and Interventions (SCAI) released a new overview on the implantation of left atrial appendage occlusion devices.
The implantation of left atrial appendage occlusion devices may lower the risk of stroke in patients with atrial fibrillation. As new devices are developed, it is anticipated that the use of left atrial appendage occlusion technologies in clinical practice will expand. The authors of the paper urge that the new technology should be disseminated thoughtfully, with emphasis on team-based care and the collection of the necessary data in longitudinal registries to determine ideal patient selection, effectiveness and safety. It will also be necessary to develop and implement new guidelines, expert consensus statements, requirements for training, operator credentialing and institutional policies.
"This document highlights the critical issues surrounding left atrial appendage occlusion therapies," said Frederick A. Masoudi, M.D., MSPH, FACC, professor of medicine at the University of Colorado Anschutz Medical Campus and chair of the writing committee. "We aimed to facilitate the alignment among patients and their families, primary care physicians, general cardiologists, and additional heart team members and procedural specialists. We also identified the need to collect robust clinical data on outcomes for patients who are treated with these devices in clinical practice, especially because in some cases, the evidence for some devices in use is sparse."
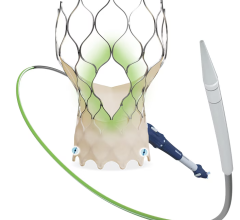
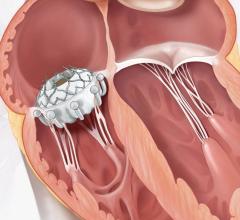
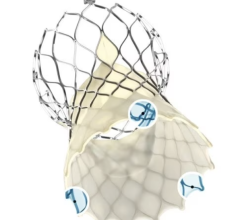
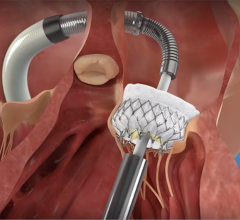
While oral anticoagulants are the standard of care to reduce risk of stroke in patients with atrial fibrillation, some of these patients have contraindications to these drugs or are unable or unwilling to adhere to long-term oral anticoagulation therapy. In these cases, left atrial appendage occlusion may be considered. The use of left atrial appendage occlusion devices is likely to increase with the recent approval by the U.S. Food and Drug Administration (FDA) of the Watchman device. Watchman was approved for patients who are at an increased risk of stroke based upon their clinical profile, are deemed suitable for anticoagulation therapy with warfarin, and have an appropriate rationale to seek a non-pharmacological alternative to warfarin therapy after taking into account the safety and effectiveness of the device.
This document is the first in a series from the ACC, HRS and SCAI that will address the integration of new technologies into the care of patients with atrial fibrillation. It will be published on the websites of all three organizations and in future issues of the Journal of the American College of Cardiology, Heart Rhythm, and Catheterization and Cardiovascular Interventions.
For more information: www.acc.org, www.hrsonline.org, www.scai.org


 July 08, 2024
July 08, 2024