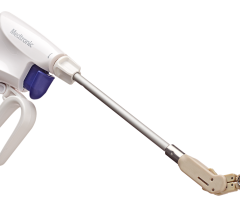
March 23, 2015 — AtriCure Inc. announced that it has launched the availability of its cryoICE cryo ablation probe (CRYO2) to provide cryoanalgesia for temporary pain management. AtriCure’s cryoICE is the first U.S. Food and Drug Administration (FDA)-cleared cryo ablation probe for the treatment of cardiac arrhythmias and for the temporary ablation of peripheral nerves to block pain.
Cryoanalgesia is a method of temporarily relieving pain by cryo ablating the affected nerve, causing an interruption of pain impulses to the brain. For cardiac and thoracic surgery patients, the pain impulses are blocked by ablating the intercostal nerve bundle(s), thereby alleviating the pain associated with a thoracotomy incision. When applied appropriately, cryoanalgesia can relieve pain for several days and in some cases weeks.
AtriCure has a portfolio of products that include radiofrequency and cryo ablation devices that are used during cardiac surgery. The company’s cryoICE cryo ablation system has been indicated to freeze target tissue for the treatment of cardiac arrhythmias. The recently expanded indication for the cryoICE probe “for use in blocking pain by temporarily ablating peripheral nerves” provides surgeons with an additional option to treat post-thoracotomy pain.
“The pain management options available today are not optimal,” stated Andrea Trescot, M.D., past president of the American Society of Interventional Pain Physicians. “Cryoanalgesia has been studied for many years, and has been shown to successfully block pain with no long-term neural effects.”
Currently, surgeons use epidurals, intercostal nerve blocks and prescription narcotics for pain management. Studies have suggested that cryoanalgesia is a useful method for both short- and long-term relief from pain related to thoracotomy access. It is estimated that more than 25,000 cardiac and thoracic surgeries are performed in the United States every year using a thoracotomy approach.
“I have used cryoanalgesia as an adjunct to conventional pain relief techniques in nearly 75 cases of mitral valve surgeries via mini-thoracotomies,” stated Francis Shannon, M.D., cardiothoracic surgeon, Beaumont Health System. “I have been very impressed with the reduction in respiratory complications after surgery as well as narcotic requirements for chest pain.”
For more information: www.atricure.com


 April 04, 2024
April 04, 2024