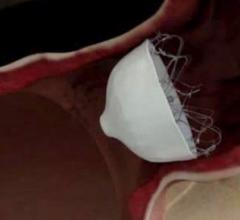
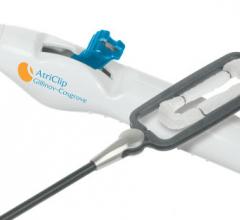
February 22, 2018 — AtriCure Inc. announced that it has launched the AtriClip FLEX•V Left Atrial Appendage (LAA) ...
Left Atrial Appendage (LAA) Occluders
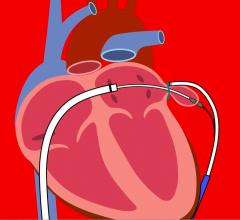
This channel includes news and new technology innovations about Left Atrial Appendage (LAA) Occluders. These close off the LAA in patients with atrial fibrillation to prevent the formation of stroke-causing clots in atrial fibrillation (AFib or AF) patients. LAA occlusion is often indicated for patients who do not tolerate anticoagulation therpy or have bleeding risks associated with use of that therapy.
January 18, 2018 – Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc., a worldwide leader ...
Dee Dee Wang, M.D., director, structural heart imaging at Henry Ford Hospital, Detroit, explains how her center uses 3-D ...
Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital ...
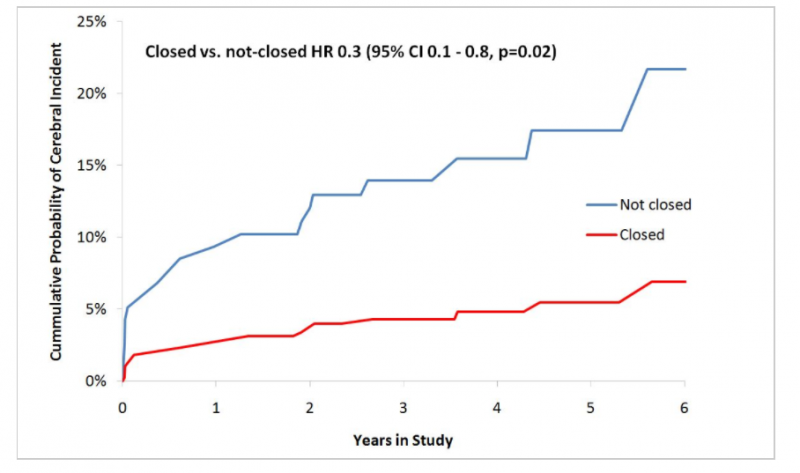
September 7, 2017 — Closure of the left atrial appendage (LAA) during heart surgery protects the brain, according to ...
April 25, 2017 — AtriCure Inc. announced it has sold more than 100,000 AtriClip Left Atrial Appendage Exclusion System ...

March 29, 2017 — For patients with atrial fibrillation (AFib), closing the area of the heart known as the left atrial ...
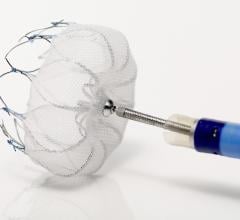
March 22, 2017 — Aegis Medical Innovations Inc. announced that it has received Investigational Device Exemption approval ...

March 3, 2017 — A Henry Ford Hospital study found a 100 percent success rate in left atrial appendage (LAA) occlusion ...
January 6, 2017 — SentreHeart Inc. announced that it has completed the Stage I enrollment milestone in the aMAZE Trial ...

November 7, 2016 – Results from the U.S. real-world, post-FDA approval experience of the Watchman device found high ...
October 25, 2016 — A recent study from University of Alabama at Birmingham (UAB) researchers published in PLOS ONE compa ...
October 20, 2016 — SentreHeart Inc. announced in late September the closing of a $35 million Series D round of financing ...
October 13, 2016 — SentreHeart Inc. announced this week it has treated the first patients using the Eclipse Surgical ...

New cardiovascular device therapies for atrial fibrillation (AF) and heart failure (HF) are rapidly evolving with the ...

 February 22, 2018
February 22, 2018