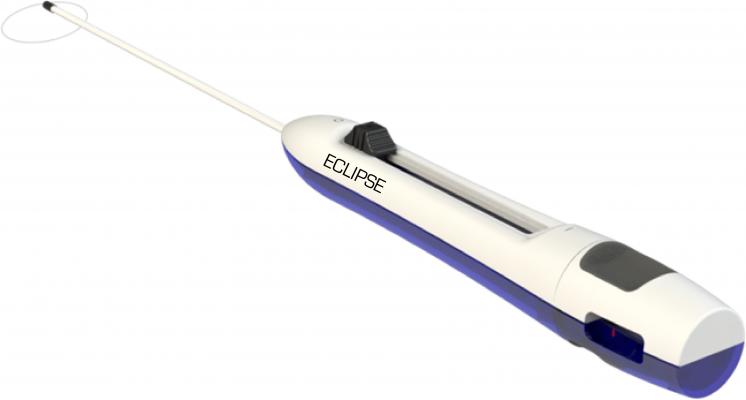
October 13, 2016 — SentreHeart Inc. announced this week it has treated the first patients using the Eclipse Surgical Device, which is approved and CE Marked in Europe for left atrial appendage (LAA) closure. Krzysztof Bartus, M.D., Ph.D., and associate professor of the Jagiellonian University, Department of Cardiovascular and Transplant Surgery in Krakow, Poland performed the procedures.
According to Bartus, “The procedures went exceedingly well. Complete closure was achieved in all cases. The ultra-low profile of the Eclipse improves visualization during placement, is easy to use and unlike other surgical solutions, does not require excessive grasping or manipulation of the left atrial appendage to close around its base.”
The Eclipse enables remote delivery of a 50mm pre-tied suture loop in a controlled and precise manner with immediate and complete LAA closure.
For more information: www.sentreheart.com


 December 19, 2025
December 19, 2025 









