October 20, 2016 — SentreHeart Inc. announced in late September the closing of a $35 million Series D round of financing. Deerfield Management Company, a healthcare investment firm, led the financing, which included participation from SentreHeart’s existing investors U.S. Venture Partners, Prospect Ventures, Vivo Capital and Decheng Capital.
The proceeds will be utilized to complete the aMAZE (alternative-MAZE) randomized, pivotal clinical trial evaluating the use of the Lariat device for closure of the left atrial appendage (LAA) as an adjunctive treatment to ablation in patients with persistent or longstanding persistent atrial fibrillation (AFib). Deerfield Partner Ted Huber will join SentreHEART’s Board of Directors.
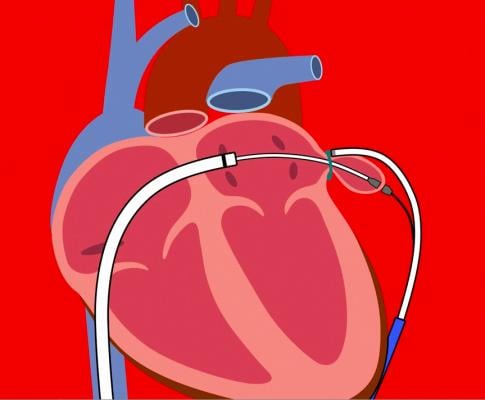
Recent studies have demonstrated that the Lariat device not only closes the LAA mechanicallyi, but can also isolate electrical activity within the LAAii, a known contributor for AFibiii. The objective of the aMAZE Trial is to demonstrate that Lariat for LAA closure, plus a pulmonary vein isolation (PVI) ablation, will lead to a reduced incidence of recurrent AFib compared to PVI alone, with a high safety profile.
The Lariat Suture Delivery Device is indicated for suture placement and knot tying in surgical procedures where soft tissues are being approximated and/or ligated with a pre-tied polyester suture. SentreHeart received U.S. Food and Drug Administration (FDA) 510(k) clearances for the device in 2006, 2009 and 2014. It also has CE Mark approval in Europe.
For more information: www.amazetrial.com
i Bartus K, et al. Percutaneous Left Atrial Appendage Suture Ligation Using the LARIAT Device in Patients with Atrial Fibrillation. J Am Coll Cardiol 2013 Jul 9;62(2):108-18.
ii Han FT, et al. The Effects of LAA Ligation on Electrical Activity. Heart Rhythm. 2014 May; 11(5):864-70.
iii Di Biase L, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010 Jul 13;122(2):109-18.


 December 19, 2025
December 19, 2025 









