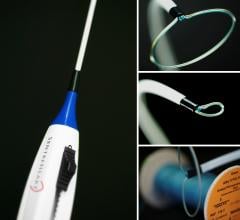
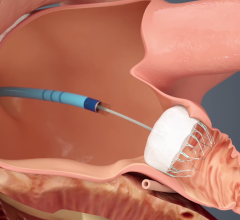
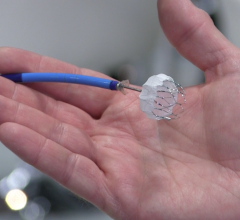
September 2, 2016 — St. Jude Medical Inc. announced the start of the St. Jude Medical Amplatzer Amulet IDE trial of the ...
Left Atrial Appendage (LAA) Occluders
This channel includes news and new technology innovations about Left Atrial Appendage (LAA) Occluders. These close off the LAA in patients with atrial fibrillation to prevent the formation of stroke-causing clots in atrial fibrillation (AFib or AF) patients. LAA occlusion is often indicated for patients who do not tolerate anticoagulation therpy or have bleeding risks associated with use of that therapy.

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...
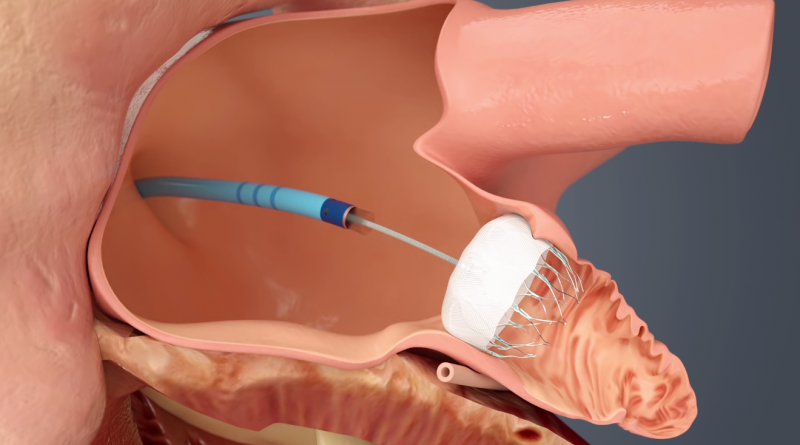
Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the ...
July 7, 2016 — AtriCure Inc. announced the first patient has been enrolled in the FROST study at William Beaumont ...
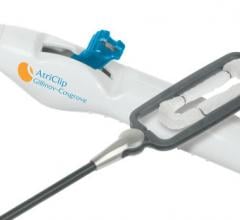
July 5, 2016 — AtriCure Inc. announced that it has received CE Mark for the AtriClip PRO2 Left Atrial Appendage (LAA) ...

Atrial fibrillation (AF) affects nearly 6 million Americans and the condition puts them at significantly greater risk of ...
May 19, 2016 — St. Jude Medical Inc. announced results from two cardiovascular clinical trials presented at EuroPCR 2016 ...
April 29, 2016 — AtriCure Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the AtriClip PRO2 ...
David Holmes, M.D., professor of medicine, Mayo Clinic College of Medicine and consultant, Department of Internal ...
March 16, 2016 — The Valley Hospital in Ridgewood, N.J., is one of 15 U.S. sites currently enrolling patients in a ...
February 18, 2016 — AtriCure Inc. announced that the first patient was enrolled and treated at PinnacleHealth Hospitals ...
February 9, 2016 — Boston Scientific Corp. announced the Centers for Medicare and Medicaid Services (CMS) will cover ...
January 6, 2016 — A new study determined that the Watchman left atrial appendage closure device is more cost-effective ...
December 29, 2015 — The American College of Cardiology (ACC) launched the new Left Atrial Appendage Occlusion (LAAO) ...
November 24, 2015 — Biosense Webster announced it has acquired Coherex Medical Inc., a privately held medical device ...


 September 02, 2016
September 02, 2016