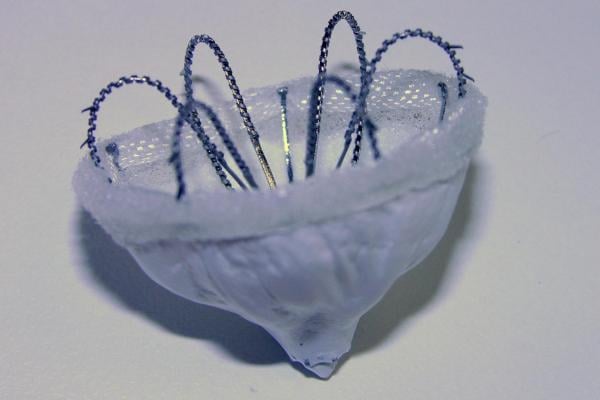
November 24, 2015 — Biosense Webster announced it has acquired Coherex Medical Inc., a privately held medical device company based in Salt Lake City, Utah focused on the development of the Coherex WaveCrest Left Atrial Appendage (LAA) Occlusion System. The device is designed to permanently occlude the LAA in high-risk atrial fibrillation (Afib) patients to help reduce the incidence of stroke caused by the migration of blood clots formed in the LAA, which is recognized as the source of blood clots in approximately 90 percent of patients who have Afib.
“The addition of the Coherex WaveCrest System complements our comprehensive portfolio of therapeutic solutions for patients suffering from atrial fibrillation who not only suffer from reduced quality of life, but also face a significantly greater risk of a stroke,” said Shlomi Nachman, company group chairman. "As the exclusive distributor of this system in regions outside of the U.S. since 2013, we are confident it will be well-differentiated in the market.”
Afib is associated with a five-fold increased risk of stroke and prevention in patients with Afib represents a large unmet clinical need. This acquisition reinforces the company's commitment to providing innovative, minimally invasive therapies for the treatment of Afib, which affects over 3 million patients in the United States and 20 million worldwide.
"The Coherex WaveCrest System offers substantial benefits for patients with atrial fibrillation who are at high risk for stroke, particularly for those who are contraindicated to anticoagulants and would therefore, be unprotected from the risk of cardio embolism," said Alex Martin, president and CEO, Coherex Medical. "We are excited that this technology will be coupled with Biosense Webster’s market leading therapies and look forward to bringing this innovative solution to more patients worldwide who stand to benefit from reduced risk of stroke.”
The Coherex WaveCrest LAA occlusion system received CE mark in September 2013. It is not available for investigational use or commercial distribution in the United States at this time.
For more information: www.biosensewebster.com


 December 19, 2025
December 19, 2025 









