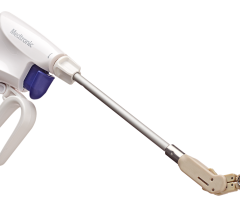
July 1, 2015 - SentreHeart Inc. announced that it has received approval for an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin enrollment in a clinical study of the Lariat Suture Delivery Device. The randomized, controlled clinical study, known as the AMAZE Trial, will evaluate the use of the Lariat device for the ligation, or closure, of the left atrial appendage (LAA) as an adjunctive treatment to ablation in patients with persistent or longstanding persistent atrial fibrillation (AFib).
"The LAA is an important site for atrial fibrillation initiation and persistence, and its exclusion using the Lariat device as an adjunct to conventional ablation could be a major breakthrough in decreasing recurrence in patients with persistent atrial fibrillation," said Dhanunjaya Lakkireddy, M.D., FACC, FHRS, professor of medicine, director, Center for Excellence in AF and Complex Arrhythmias, University of Kansas Medical Center. "The AMAZE trial is rigorously designed and we believe will further validate the mechanical and electrical isolation benefits of the Lariat device, which has the potential to become a standard of care in treating persistent or longstanding persistent atrial fibrillation."
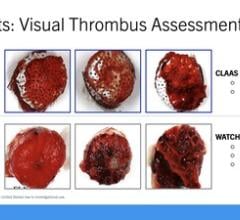
Recent studies have demonstrated that the device not only closes the LAA mechanically but can also isolate electrical activity within the LAA, a known trigger for AFib. The objective of the AMAZE Trial is to demonstrate that the Lariat for LAA closure, plus a pulmonary vein isolation (PVI) ablation, will lead to a reduced incidence of recurrent AFib compared to PVI alone, with a high safety profile.
The study is comprised of two stages. The overall study plan is to enroll a maximum of 600 persistent or longstanding persistent AFib patients who are candidates for PVI catheter ablation at up to 50 centers. The first stage of the AMAZE Trial will enroll up to 175 patients at 15 centers.
"The left atrial appendage has been accurately termed our most lethal human attachment," stated James L. Cox, M.D., the surgeon who developed the gold-standard Cox-maze procedure as a cure for AFib. "The Lariat is the only percutaneous device that can provide the electromechanical isolation of the myocardium of the LAA by devascularization and, when combined with PVI ablation, would seem to be the one most likely to improve catheter ablation outcomes for AFib."
PVI catheter ablation is the standard of care interventional treatment for patients with persistent and longstanding persistent AFib; however, not all electrical activity originates from the pulmonary veins. The LAA has been known to play a role in triggering recurrence of AFib after treatment with PVI catheter ablation, and is the source of most stroke-causing blood clots (thrombus) in AFib patients.
The Lariat Suture Delivery Device is indicated for suture placement and knot tying in surgical procedures where soft tissues are being approximated and/or ligated with a pre-tied polyester suture. SentreHEART received FDA 510(k) clearances for the Lariat in 2006, 2009 and 2014. The device also has CE Mark approval in Europe.
For more information: www.sentreheart.com


 April 04, 2024
April 04, 2024