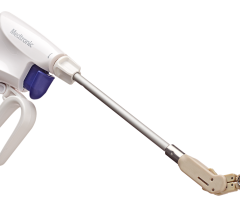
Image courtesy of SentreHeart
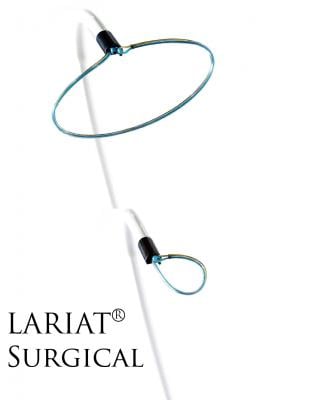
October 28, 2015 — SentreHeart Inc. announced that it has received CE Mark approval for the Lariat Surgical Left Atrial Appendage (LAA) Suture Delivery Device. The Lariat Surgical LAA device is a suture-based solution for soft tissue closure, including the LAA. European surgeons can now offer their patients precise, user-controlled delivery of a 50mm pre-tied suture loop through traditional open surgical procedures or through an access port as small as 5mm.
The device compliments the company’s innovative soft tissue closure technology by leveraging the Lariat’s low-profile delivery, designed to be compatible with a broad array of surgical approaches for LAA or other soft tissue closure. Features of the Lariat include an easy-to-use 50mm snare that is compatible with access as small as 5mm, a malleable shaft design for ease of use in delivery from different access locations and an integrated suture-tightening system to reduce the risk of operator variability during closure. The surgeon, while under direct visualization, can guide the snare loop over soft tissue, including the left atrial appendage, confirm its exact closure location and deploy the pre-tied suture, resulting in immediate, complete ligation without leaving any metal or clip behind.
Unlike current surgical closure solutions, including staplers and clips, the Lariat Surgical LAA device uses a common polyester suture, which may prevent any metal-to-tissue interaction. Additionally, its low-profile design will give surgeons greater control in suture placement location and is designed to be compatible with open-chest, thoracotomy or port access procedures.
Krzysztof Bartus, M.D., Ph.D., associate professor of cardiac surgery at Jagiellonian University Krakow, Poland stated, “The Lariat Surgical device is an elegant surgical closure solution, combining the best of all the current approaches into a single device. The new device enables flexibility in my approach and access to close tissue where I want, with the additional confidence that it will remain closed. These are important characteristics when closing soft tissue such as the left atrial appendage.”
For more information: www.sentreheart.com


 April 04, 2024
April 04, 2024