July 31, 2024 — A novel study co-authored by a heart failure cardiologist at University Hospitals Harrington Heart & ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
As part of DAIC's continuing Thought Leadership Series, this month Editorial Director Melinda Taschetta-Millane sits ...
July 30, 2024 — Patients with congestive heart failure (CHF) having a compromised blood supply, are at greater risk of ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
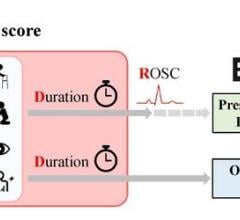
July 29, 2024 — When it comes to treating cardiac arrest, acting quickly can mean the difference between life and death ...
July 29, 2024 — Edwards Lifesciences announced investments that reflect the company’s deep commitment to advancing ...
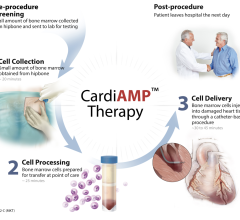
July 25, 2024 — BioCardia, Inc., a global leader in cellular and cell-derived therapeutics for the treatment of ...
>July 24, 2024 — Increasing ketone supply to the heart in mice with heart failure with preserved ejection fraction ...
July 23, 2024 — The International Atherosclerosis Society (IAS) has released a clinical proceedings white paper ...
July 18, 2024 — AliveCor, a global leader in AI-powered cardiology, today announced that the American Medical ...
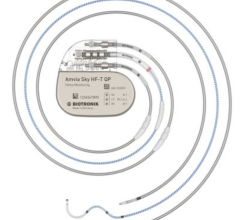
July 11, 2024 — Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P ...
July 11, 2024 — Heart Test Laboratories, Inc. an artificial intelligence (AI)-powered medical technology company focused ...
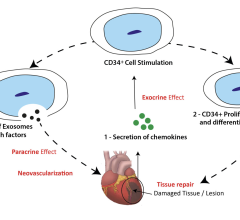
July 10, 2024 — CellProthera, a private company specializing in cell-based therapies for repairing ischemic tissues, and ...
July 9, 2024 — Disparities in cardiovascular disease outcomes between urban and rural areas continue to widen, yet ...

From FDA approvals to new clinical trials, here is a look at DAIC's top-read content during the month of June:
1. Med ...
July 1, 2024 — CVRx, Inc., a commercial-stage medical device company, announced three additions to its senior leadership ...


 July 31, 2024
July 31, 2024