April 9, 2024 — The investigational drug ninerafaxstat showed a good tolerability and safety profile, along with evidence of improvements in symptoms and exercise capacity among people with nonobstructive hypertrophic cardiomyopathy (HCM), according to results of a 12-week phase 2 trial presented at the American College of Cardiology’s Annual Scientific Session.
Ninerafaxstat is designed to help the heart work more efficiently by altering how the muscle uses energy, partially inhibiting the use of fatty acids as fuel and encouraging the use of glucose instead. While the IMPROVE-HCM trial focused primarily on tolerability and safety of the drug, depending on outcomes of future clinical trials, it could help people with nonobstructive HCM overcome fatigue, chest discomfort and other activity-limiting symptoms of the condition, for which there are few existing treatments.

Martin S. Maron, MD
“Our findings provide enthusiasm that a novel drug therapy with ninerafaxstat may provide nonobstructive HCM patients an opportunity to achieve a better quality of life by decreasing symptom burden and improving exercise capacity,” said Martin S. Maron, MD, a cardiologist and director of the Hypertrophic Cardiomyopathy Center at Lahey Hospital and Medical Center in Burlington, Massachusetts, and the study’s lead author. “The safety and tolerability of the drug in this phase 2 trial was excellent. These data strongly support moving on to phase 3 development.”
HCM is the most common genetic heart disease worldwide and is estimated to affect 1 in 500 people. It causes the heart muscle to become stiff and thick, making it harder for the heart to pump blood properly and increasing the risk of sudden cardiac death. Several treatment options are available for the approximately two-thirds of patients who have the obstructive form of the disease, in which the thickening muscle makes it harder for blood to flow out of the heart, but there are limited treatment options for the remaining one-third of patients who have the nonobstructive form.
Most people with nonobstructive HCM have no symptoms, but about 40% experience symptoms such as chest pain, shortness of breath, abnormal heart rhythms, dizziness, fainting and swelling, which often worsen over time.
“There’s little question that the greatest unmet treatment need for HCM is in the nonobstructive patients who experience limiting symptoms. They’re very frustrated by the impact of symptoms on their quality of life with no approved medical therapies,” Maron said.
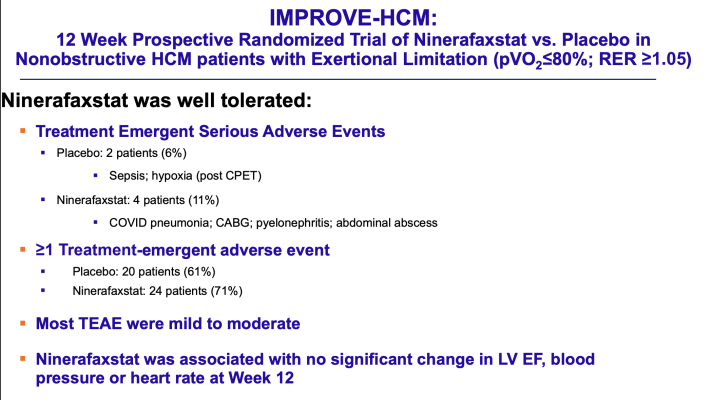
For the phase 2 trial, researchers enrolled 67 patients treated for nonobstructive HCM at 14 centers. Participants were randomly assigned to receive either ninerafaxstat or a placebo for 12 weeks.
At the end of this period, the researchers found no significant difference in the rate of adverse events among the two study groups, indicating the drug was not associated with any increased risk of adverse events. There were also no adverse events related to low ejection fraction (a measure of how well the heart pumps blood) and no adverse effects on blood pressure or heart rate.
In addition, patients taking the drug showed improvements in secondary endpoints including ventilatory efficiency—a measure of the heart’s performance during exercise—and decreased size of the left atrium, which provides evidence that the drug worked the way it was designed to. On Kansas City Cardiomyopathy Questionnaires (KCCQ), an established method for assessing quality of life for people with heart problems, there was no difference between the two study groups overall, but there was a nine-point improvement among the subset of participants taking ninerafaxstat who reported having significant symptoms at the start of the study compared with symptomatic patients who took a placebo.
“When you confine the analysis of the study to patients who at baseline were particularly symptomatic, there was a significant improvement in the KCCQ scores for those patients compared to placebo, indicating that the drug was making those patients feel much better,” Maron said.
As a phase 2 trial, the study had a relatively small sample size and was designed primarily to assess the drug’s tolerability and safety. Maron said that a larger phase 3 trial would help shed light on the magnitude of the drug’s potential benefits. In addition, separate clinical trials are underway to assess the drug’s potential benefits for people with angina (chest pain) and heart failure with preserved ejection fraction.
The study was funded by Imbria Pharmaceuticals, developer of ninerafaxstat. This study was simultaneously published online in the Journal of the American College of Cardiology at the time of presentation. For more information on hypertrophic cardiomyopathy, visit CardioSmart.org/HCM.


 July 31, 2024
July 31, 2024 









