January 3, 2022 — The U.S. Food and Drug Administration (FDA) recently revised its Janssen COVID-19 Vaccine Fact Sheets ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
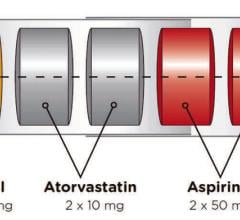
December 16, 2021 – Spanish pharmaceutical company Ferrer and the Spanish National Center for Cardiovascular Research ...

The following are the late-breaking science presentation sessions at the 2021 American Heart Association (AHA) 2021 ...
November 23, 2021 — The antiplatelet drug class of P2Y12 inhibitors that block the activity of platelets and prevents ...
November 22, 2021 — Fewer than 10 days of low-molecular-weight heparin (LMWH) after stenting for extensive iliofemoral ...
Susan Cheng, M.D., MPH, director of the Institute for Research on Healthy Aging in the Department of Cardiology at ...
November 19, 2021 — Adults treated with empagliflozin during hospitalization for acute heart failure experienced a ...
November 19, 2021 — A high dose of a purified omega-3 fatty acid, available by prescription only, was well tolerated ...
November 19, 2021 — Results from two early clinical studies show the first version of an oral PCSK9-inhibitor ...
November 18, 2021 — Moderna Inc. announced positive data from the AstraZeneca-led Phase 2 (EPICCURE) study evaluating ...
November 18, 2021 — People who are on the antiplatelet medication ticagrelor and need coronary artery bypass surgery may ...
November 17, 2021 — Taking daily low-dose aspirin for seven years did not affect the risk of dementia or mental decline ...
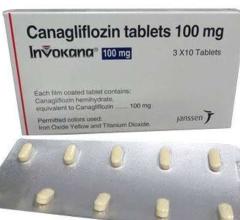
November 17, 2021 — Canagliflozin, a medication used to treat type 2 diabetes, was found to greatly improve symptoms and ...
November 15, 2021 — Novartis announced results from a pooled post-hoc analysis of Phase III ORION-9, -10 and -11 trials ...

 January 03, 2022
January 03, 2022