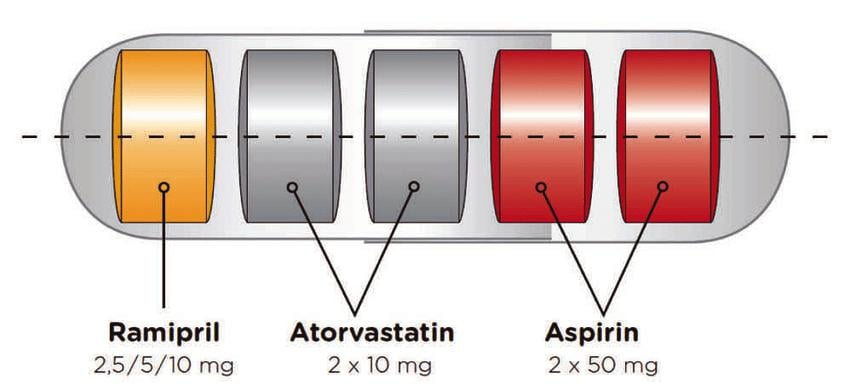
The Fuster-CNIC-Ferrer cardiovascular polypill combines three drugs into one pill to make it easier for for patients to comply with secondary cardiovascular prevention therapy.
December 16, 2021 – Spanish pharmaceutical company Ferrer and the Spanish National Center for Cardiovascular Research (Centro Nacional de Investigaciones Cardiovasculares — CNIC), announced the results of NEPTUNO, the first real-world clinical study on patients treated with the CNIC-Polypill. This single pill, composed of the three class A medications recommended in the cardiovascular prevention guidelines,[1] is used to treat patients who have previously suffered a cardiovascular (CV) event, such as heart-attack or ischemic stroke.
The NEPTUNO clinical study for secondary cardiovascular prevention was carried out on 6,456 patients in Spain. Most had one previous cardiovascular event; approximately ten per cent had experienced two. The study’s purpose was to determine the effectiveness of the CNIC-Polypill in the incidence of MACE (major adverse cardiovascular events) in secondary prevention patients, within the context of routine clinical practice. Additionally, the effectiveness on cardiovascular risk factors (CVRF) such as blood pressure and lipidic profile (total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides) was assessed. Persistence to therapy was also investigated as well as the utilization of healthcare resources and costs.
Significant Real-life Data Results From the NEPTUNO Study
The results from the NEPTUNO study demonstrate a decreased risk of MACE in CNIC-Polypill treated patients, when compared to those being administered identical or equipotent drugs separately. Patients treated with the CNIC-Polypill also showed improved control of CVRF, such as blood pressure and lipid profile, together with a longer persistence to therapy, when compared to patients taking the identical or equipotent drugs separately.[2] The study demonstrates that the use of the CNIC-Polypill significantly lowers the demand on healthcare resources, when compared to control cohorts. This reduction in utilization and associated costs is significant in terms of patient hospitalization days, resulting in lower CV events and a reduction in the number of medical visits required in the CNIC-Polypill cohort.[3] The results of the NEPTUNO study were presented at the European Society of Cardiology (ESC) Congress 2021 at the end of August.[2,3]
“With these results, Ferrer continues to show a strong commitment to improving patients’ lives within the cardiovascular sector, reducing recurrent MACE. We aim to provide a baseline treatment for patients who have suffered heart attacks and/or ischemic strokes - to avoid recurrent CV events, hence the importance of these results," said Rodrigo Palma dos Reis, chief medical officer at Ferrer.
This study is the first direct observation of the real-world impact and outcomes of the CNIC-Polypill for secondary prevention patients within healthcare settings. The CNIC-Polypill is the result of a co-development and partnership between CNIC (Centro Nacional de Investigaciones Cardiovasculares, the Spanish National Center for Cardiovascular Research, Madrid) and Ferrer.
To confirm these results, a randomized clinical trial is under way: SEcondary prevention of CardiovascUlaR disease in the Elderly (SECURE).[4] Results of the analysis of 2,500 patients are expected to be presented at the 2022 ESC Congress in Barcelona. These will focus on hard outcomes, when compared to standard therapy. They will be presented by Dr. Valentí Fuster, general director at CNIC and Dr. José Maria Castellano, coordinator of clinical trials.
Going-forward, the CNIC-Polypill could be a useful clinical strategy - as baseline therapy - to reduce the incidence of new CV events and improve the control of CVRF in patients in secondary cardiovascular prevention settings.
Polypill Developed to Make it Easier to Address Cardiovascular Disease Prevention
According to the World Health Organization, CardioVascular Disease (CVD) causes more than half of all deaths across Europe, where CVD causes 46 times the number of deaths and 11 times the disease burden than that of AIDS, TB and malaria combined. 80% of premature heart disease and strokes are preventable.[5]
According to the ESC, nearly 49 million people are living with the disease in Europe, and each year, CVD causes 3.9 million deaths in Europe.[6]
It has been estimated that up to 50-75% of patients with previous myocardial infarction will have a recurrent cardiovascular event in the same or different vascular beds within 1-3 years, respectively, after the acute cardiac event.[7-9] In addition, patients diagnosed with prior myocardial infarction have a five- to six-fold higher risk for cardiovascular death, within the first year.[10]
The pharmaceutical form is a hard capsule that contains six different possible formulations - according to the dosage of the three components of the polypill, which are Acetyl Salicylic Acid (ASA), Atorvastatin (A) and Ramipril (R).
The NEPTUNO study is a retrospective, non-interventional analysis of an anonymized medical electronic dataset of the BIG-PAC administrative database covering 2015-2018; it contains anonymized data of 1.8 million clinical histories of patients from seven primary care and hospital areas in Spain
For more information: www.cnic.es, www.ferrer.com
References:
1. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Aug 30:ehab484. doi: 10.1093/eurheartj/ehab484
7. Andres E, Cordero A, Magan P, Alegria E, Leon M, Luengo E, et al. Longterm mortality and hospital readmission after acute myocardial infarction: an eight-year follow-up study. Rev Esp Cardiol. 2012;65:414-20
8. Soldati S, Di Martino M, Castagno D, Davoli M, Fusco D. In-hospital myocardial infarction and adherence to evidence-based drug therapies: a real-world evaluation. BMJ Open. 2021;11:e042878
9. Zabawa C, Cottenet J, Zeller M, Mercier G, Rodwin VG, Cottin Y, et al. Thirty-day rehospitalizations among elderly patients with acute myocardial infarction: Impact of postdischarge ambulatory care. Medicine (Baltimore). 2018;97:e11085
10. Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull World Health Organ. 2005;83:820–9


 January 05, 2026
January 05, 2026 









