June 30, 2021 — Abbott announced its Xience family of drug-eluting coronary stents received U.S. Food and Drug ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
June 28, 2021 - A recent analysis found that children and adolescents with multisystem inflammatory syndrome in children ...
June 24, 2021 — Data captured in American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) regis ...
June 21, 2021 — The U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim's dabigatran etexilate ...
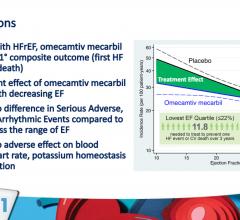
May 18, 2021 — The experimental heart failure drug omecamtiv mecarbil reduced heart failure hospitalizations by a ...
May 18, 2021 — Dapagliflozin (Farxiga), a sodium-glucose co-transporter 2 (SGLT2) inhibitor, did not significantly ...
May 17, 2021 — The anticoagulant rivaroxaban (Xarelto), in addition to low-dose aspirin, significantly reduced the ...

May 16, 2020 — New data from in the NODE-301 Trial for the patient-administered nasal spray etripamil to resolve ...
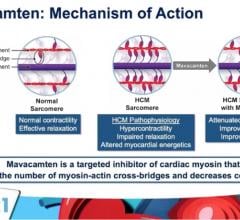
May 15, 2021 — A new analysis of the Phase 3 EXPLORER-HCM study evaluating mavacamten, an investigational, first-in ...
May 15, 2021 — The combination heart failure drug sacubitril/valsartan (Entresto) did not significantly reduce the rate ...
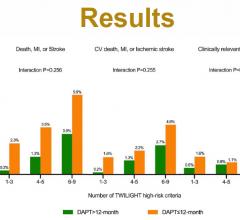
May 15, 2021 — The ADAPTABLE trial found no significant differences in cardiovascular events or major bleeding in ...
May 15, 2021 — The anticoagulant apixaban (Eliquis) was not superior to standard of care following transcatheter aortic ...
April 28, 2021 — An analysis of the prospective Chinese Fuwai PCI Registry, confirms long-term, dual-antiplatelet ...
(This story was updated May 7, 2021 in the last subheaded section)
April 13, 2021 — The U.S. Food and Drug ...
April 6, 2021 — Abbott today announced its Xience stent has received CE mark in Europe for shorter duration of dual anti ...


 June 30, 2021
June 30, 2021





![Comparison showing platelet adhesion to the surface of various coronary artery drug-eluting stents (DES) in a preclinical study that used aspirin only. Abbott said the Xience stent's fluoropolymer is significantly more anti-thrombotic than other DES.[2]](/sites/default/files/styles/content_feed_medium/public/DES_Comparison_thrombus_formation_Stents_Abbott.jpg?itok=mfh9GUz-)