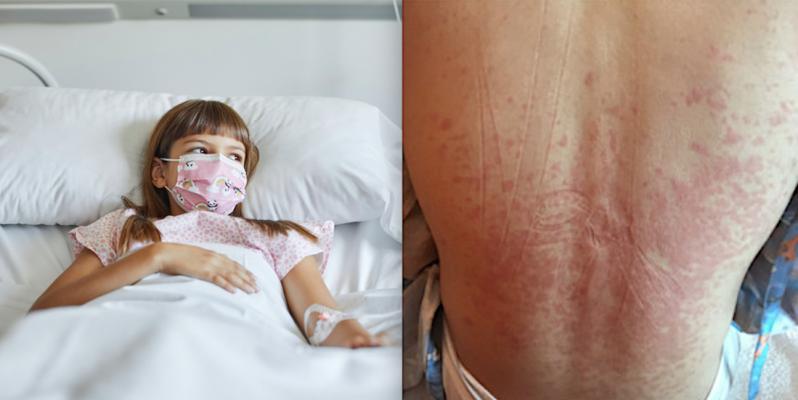
Researchers analyzing surveillance data on 518 children and adolescents with MIS-C who were admitted to U.S. hospitals in 2020 fopiund those treated with intravenous immune globulin (IVIG) plus glucocorticoids had a lower risk of new or persistent cardiovascular dysfunction than IVIG alone. Left photo Getty Images, right photo Nemours Children’s Health System.
June 28, 2021 - A recent analysis found that children and adolescents with multisystem inflammatory syndrome in children (MIS-C) caused by COVID-19 initially treated with intravenous immune globulin (IVIG) plus glucocorticoids had a lower risk of new or persistent cardiovascular dysfunction than IVIG alone. The research was part of the Overcoming COVID-19 Study, a nationwide collaboration of physicians at pediatric hospitals and the Centers for Disease Control and Prevention (CDC).
The results were published online in the New England Journal of Medicine.[1]
The researchers analyzed surveillance data on 518 children and adolescents with MIS-C who were admitted to U.S. hospitals between March 15 and Oct. 31, 2020. Eighty-nine (17%) received IVIG only; 241 (47%) received IVIG and glucocorticoids; 107 (21%) received IVIG, glucocorticoids, and a biologic; and 81 (16%) received other treatments, including glucocorticoids only, a biologic only, glucocorticoids and a biologic, or IVIG and a biologic.
They found that initial treatment with IVIG plus glucocorticoids (103 patients) was associated with a lower risk of cardiovascular dysfunction on or after day two than IVIG alone — 103 patients or 17 vs. 31%. Among those who received IVIG plus glucocorticoids, left ventricular dysfunction occurred in 8% and 17% of the patients, respectively, and shock resulting in vasopressor use in 13% and 24%. The use of adjunctive therapy was also lower among patients who received IVIG plus glucocorticoids than among those who received IVIG alone — 34 vs. 70%.
"Because MIS-C cases have been sporadic, following surges of COVID-19 cases, we haven't had the benefit of randomized clinical trials of treatment strategies," explained Tamara Bradford, M.D., associate professor of pediatrics at Louisiana State University (LSU) Health New Orleans School of Medicine, who practices at Children's Hospital New Orleans and was involved with the study.
The authors write that evaluating clinical outcomes in patients with MIS-C who were treated with various immunomodulatory therapies could provide insight into their effectiveness. Until published data that define best practices are available, these data provide clinicians with additional evidence to guide treatment for MIS-C. The ongoing transmission of SARS-CoV-2 and the emergence of variants of concern may promote continued outbreaks of MIS-C in the United States and internationally. Additional evidence-based studies are needed to examine the generalizability of these findings across a broad range of geographic regions and practice settings.
The Centers for Disease Control and Prevention defines MIS-C as a condition where different body parts can become inflamed, including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs. Children with MIS-C may have a fever and various symptoms, including abdominal pain, vomiting, diarrhea, neck pain, rash, bloodshot eyes, or feeling extra tired. We do not yet know what causes MIS-C. However, many children with MIS-C had the virus that causes COVID-19 or had been around someone with COVID-19.
As of June 2, 2021, a total of 4,018 patients in the U.S. have met the MIS-C case definition, with 36 deaths. Of those, 100-149 have been in Louisiana.
The study was supported by the Centers for Disease Control and Prevention under a contract with Boston Children's Hospital.
Find additional CDC statistics on U.S. MIS-C cases
Related Content on MIS-C:
Kawasaki-like Inflammatory Disease Affects Children With COVID-19
VIDEO: Overview of Multisystem Inflammatory Syndrome in Children (MIS-C) in COVID-19 Exposed Children — Interview with Deepika Thacker, M.D.
Case Study Describes One of the First U.S. Cases of MIS-C
NIH-funded Project Wants to Identify Children at Risk for MIS-C From COVID-19
New Study Looks at Post-COVID-19 Emerging Disease in Children
The Cardiovascular Impact of COVID-19
VIDEO: Example of a Multisystem Inflammatory Syndrome in Children (MIS-C) Pediatric Echocardiogram
NIH-funded Project Wants to Identify Children at Risk for MIS-C From COVID-19
Reference:


 January 05, 2026
January 05, 2026 









