December 14, 2022 — Verseon announced that the European Journal of Medicinal Chemistry has published a paper describing ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
December 9, 2022 — Imbria Pharmaceuticals, Inc., a clinical stage, cardiometabolic company developing novel therapies ...
December 8, 2022 — Esperion announced that the landmark Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regim ...
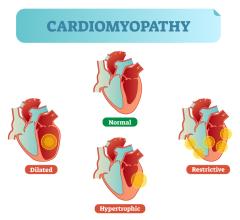
November 28, 2022 – Tenaya Therapeutics, a clinical-stage biotechnology company with a mission to discover, develop and ...
November 14, 2022 — Bivalirudin is a safer and more effective anticoagulant than heparin for treating patients with the ...
October 17, 2022 — Global Health has paid a deadly price for not using simple, low-cost blood pressure lowering drugs ...
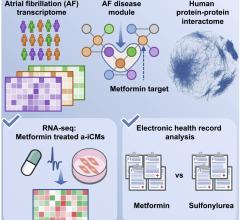
October 12, 2022 — Cleveland Clinic researchers have identified a common diabetes medication, metformin, as a possible ...
September 6, 2022 — An analysis of more than 12,000 patients has found that the SGLT2 inhibitors dapagliflozin and empag ...
September 6, 2022 — Stopping statin treatment early could substantially reduce lifetime protection against heart disease ...
August 30, 2022 — A pill containing aspirin and medications to lower lipids and blood pressure more effectively prevents ...
As the cardiology community commemorates the importance of heart health on World Heart Day, Sept. 29, here’s a quick ...
July 25, 2022 DalCor Pharmaceuticals announced the results of the dal-GenE Phase 3 cardiovascular precision medicine ...
May 24, 2022 — Amarin Corporation plc announced that research on the potential population health impact and cost ...
May 24, 2022 — The U.S. Food and Drug Administration (FDA) has issued a statement that Teva Pharmaceuticals USA has ...
May 17, 2022 — Amarin Corporation plc announced that research on the potential population health impact and cost ...

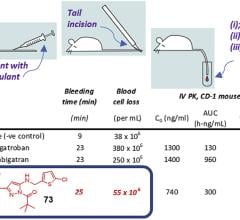
 December 14, 2022
December 14, 2022