May 2, 2023 — Sequana Medical NV, a pioneer in the treatment of fluid overload in liver disease, heart failure and ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.

As we head into Spring, let's take a look at the Top 10 topics that viewers were reading about during the month of April ...
April 28, 2023 — Windtree Therapeutics, Inc. is a biotechnology company focused on advancing late-stage interventions ...
April 25, 2023 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing late-stage interventions for ...
April 14, 2023 — A new twist on a drug used to treat alcohol use disorder could double up as a treatment for stroke, the ...
April 3, 2023 — Esperion announced the full results from the landmark Cholesterol Lowering via Bempedoic acid, an ACL ...
April 3, 2023 — Sequana Medical NV, a pioneer in the treatment of fluid overload in liver disease, heart failure and ...
March 29, 2023 — Cardiotoxicity is a clinical condition that arises from using pharmaceutical agents such as antibiotics ...
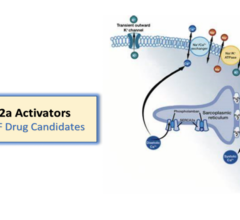
March 27, 2023 — Tenax Therapeutics, Inc., a specialty pharmaceutical company focused on identifying, developing and ...
March 24, 2023 — According to the U.S. Food and Drug Administration (FDA), Ascend Laboratories LLC is voluntarily ...
March 22, 2023 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing multiple late-stage ...
March 15, 2023 — Windtree Therapeutics, Inc, a biotechnology company focused on advancing late-stage interventions for ...
March 6, 2023 — Patients with pulmonary arterial hypertension (high blood pressure in the arteries that supply the lungs ...
March 6, 2023 — A multifaceted intervention to improve prescribing practices increased the likelihood that patients with ...
February 27, 2023 — Amarin Corporation plc announced that data evaluating the role of VASCEPA /VAZKEPA (icosapent ethyl) ...


 May 02, 2023
May 02, 2023