May 17, 2024 — The study design and baseline characteristics of FINE-HEART, a new prespecified, exploratory pooled ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
May 14, 2024 — One of the most common genetic heart diseases worldwide, hypertrophic cardiomyopathy (HCM) causes the ...
May 14, 2024 — An ambitious, nationwide clinical trial led by UVA Health’s Karen Johnston, MD, has provided doctors with ...
May 6, 2024 — Tenax Therapeutics, Inc., a Phase 3, development-stage pharmaceutical company focused on identifying ...
April 30, 2024 — A multicenter study led by Vanderbilt University Medical Center (VUMC) and Lipscomb University College ...
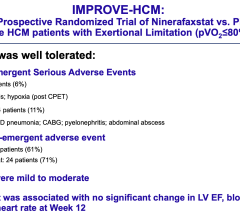
April 9, 2024 — The investigational drug ninerafaxstat showed a good tolerability and safety profile, along with ...
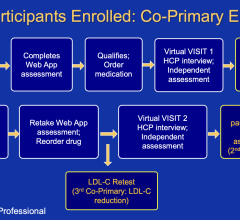
April 9, 2024 — Using a web application to qualify individuals for treatment with a nonprescription statin closely ...
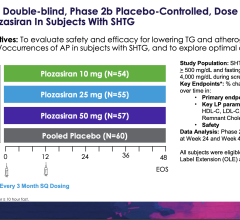
April 7, 2024 — In patients with severely elevated triglyceride levels at risk for developing acute pancreatitis, the ...
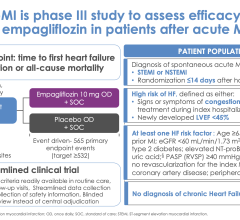
April 6, 2024 — Use of the sodium glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin following a heart attack did ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...
April 1, 2024 — Esperion announced that the United States Food and Drug Administration (FDA) has approved broad new ...
March 26, 2024 — Bristol Myers Squibb has announced that it will present data on CAMZYOS (mavacamten) for NYHA class II ...
March 13, 2024 — Silence Therapeutics has announced positive topline 36-week data from the ongoing ALPACAR-360 phase 2 ...
March 8, 2024 — Novo Nordisk today announced that the U.S. Food and Drug Administration (FDA) has approved an additional ...
February 16, 2024 — AMO Pharma Limited, a privately held clinical-stage specialty biopharmaceutical company focusing on ...

 May 17, 2024
May 17, 2024