September 2, 2022 — Dapagliflozin reduces the risk of cardiovascular death or worsening heart failure in heart failure patients with mildly reduced and preserved ejection fraction, according to late breaking research presented in a Hot Line session at ESC Congress 2022.1
Professor Scott Solomon of Brigham and Women's Hospital, Harvard Medical School, Boston, US said: “Taken together with previous research in heart failure patients with reduced ejection fraction,2 these data suggest that dapagliflozin is effective regardless of ejection fraction and support the use of SGLT2 inhibitors as foundational therapy in all patients with heart failure.”
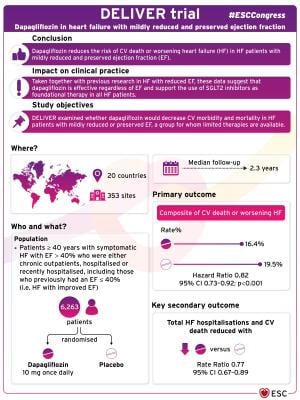
Previous studies have shown that the SGLT2 inhibitor dapagliflozin reduces cardiovascular mortality and morbidity in patients with diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.2-4 DELIVER was designed to determine whether dapagliflozin would decrease cardiovascular morbidity and mortality in patients with heart failure with mildly reduced or preserved ejection fraction, a group for whom limited therapies are available.5,6
DELIVER was a randomized, double-blind, placebo-controlled trial conducted at 353 sites in 20 countries. The trial enrolled patients aged 40 years and above with symptomatic heart failure with an ejection fraction of greater than 40% who were either chronic outpatients, hospitalized or recently hospitalized, including patients who previously had an ejection fraction of 40% or below (i.e. heart failure with improved ejection fraction). Patients were randomized to dapagliflozin or placebo and followed for a median of 2.3 years. The primary endpoint was a composite of cardiovascular death or worsening heart failure.
A total of 6,263 patients were randomly allocated to dapagliflozin, 10mg once daily, or placebo. The average age of participants was 72 years and 44% were women. The average left ventricular ejection fraction was 54%, and 18% of patients previously had an ejection fraction of 40% or less. At randomization, 77% of patients were taking an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB) or angiotensin receptor neprilysin inhibitor (ARNI), 83% were taking a beta blocker and 43% were taking a mineralocorticoid receptor antagonist (MRA).
Over a median 2.3 years, the primary outcome occurred in 512 of 3,131 patients (16.4%) in the dapagliflozin group and 610 of 3,132 patients (19.5%) in the placebo group (hazard ratio [HR] 0.82; 95% confidence interval [CI] 0.73–0.92; p<0.001).
Regarding components of the primary endpoint, worsening heart failure occurred in 368 patients (11.8%) in the dapagliflozin group and 455 patients (14.5%) in the placebo group (HR 0.79; 95% CI 0.69–0.91), and cardiovascular death occurred in 231 (7.4%) and 261 (8.3%) patients, respectively (HR 0.88; 95% CI 0.74–1.05).
Key secondary outcomes were also reduced in patients receiving dapagliflozin compared with placebo, including total heart failure hospitalizations and cardiovascular death (rate ratio 0.77; 95% CI 0.67–0.89), and total symptom burden, assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) (mean difference in the KCCQ total symptom score 2.4; 95% CI 1.6–3.2).
Professor Solomon said: “We found that dapagliflozin significantly reduced the primary composite endpoint by 18%, with numerically lower rates of all components of the primary endpoint. These benefits were consistent across prespecified subgroups, with similar benefits in patients with ejection fraction at, below, or above 60%, those with heart failure with improved ejection fraction, as well as in patients who were hospitalized recently, and was accompanied by improvement in symptoms.”
For more information: www.escardio.org
References and Notes
1DELIVER will be discussed during:
Hot Line Session 4 on Saturday 27 August at 16:30 to 17:30 CEST in the Barcelona auditorium.
Meet the Trialist – DELIVER on Sunday 28 August at 11:30 to 11:50 CEST on the ESC TV Stage.
2McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
3Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357.
4Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446.
5Solomon SD, de Boer RA, DeMets D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–1225.
6Solomon SD, Vaduganathan M, Claggett BL, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10:184–197.


 August 29, 2025
August 29, 2025