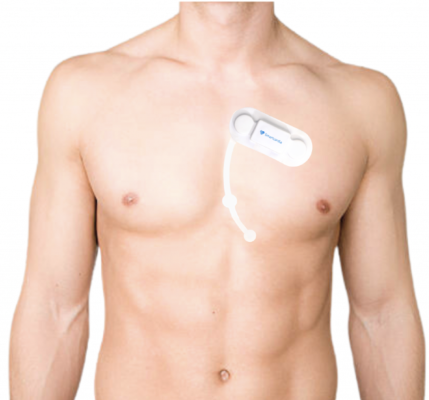
September 19, 2023 — SmartCardia has received FDA clearance for its 7-lead real-time ECG monitoring patch and cloud platform. SmartCardia's 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days.
"SmartCardia's 7L patch and cloud platform is a single solution that covers the entire spectrum of cardiac monitoring including screening, post-operative follow-up, and remote patient monitoring. It has been designed ground-up to meet the stringent requirements of these different applications", said Srinivasan Murali, co-founder and CEO of SmartCardia.
One of the major limitations of cardiac patches in the market is the lack of multiple, reciprocal leads that enable accurate arrhythmia detection. SmartCardia's 7L patch provides excellent P and QRS waves, unprecedented signal quality and allows for accurate detection of arrhythmias thanks to its 7 ECG leads.
According to the CTO, Francisco Rincon: "100% of the ECG data, not just events, is transmitted in real-time, and the automated analysis is performed throughout the entire signal. While full-disclosure analysis allows for highly accurate arrhythmia detection, easy navigation on the cloud platform allows the clinician to quickly view and analyze ECG at any point in time."
The 7L platform features real-time view of the patient's ECG and can deliver visual and audio alarms. It also provides automatic trigger of abnormal events with notifications to clinicians, and analysis of a comprehensive set of arrhythmias.
The patch and cloud platform are CE Class IIa approved. SmartCardia is expanding its global footprint with widespread adoption in Europe and India and quickly reaching new geographies. The company has been recognized by Frost & Sullivan with the 2022 Global New Product Innovation Award in cardiac monitoring.
For more information: www.smartcardia.com


 January 15, 2026
January 15, 2026 









