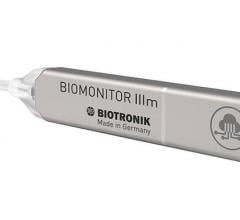
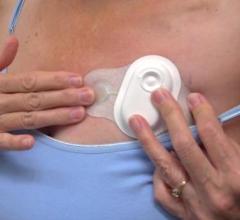
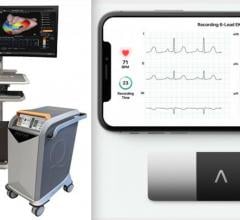
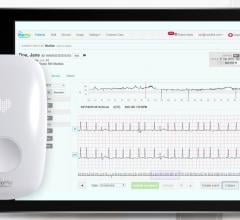
September 28, 2021 — Biotricity Inc., a medical diagnostic and consumer healthcare technology company, has developed a ...
Remote Monitoring
September 8, 2021 — Continuous heart rhythm monitoring – with anticoagulation if atrial fibrillation is detected – does ...
September 7, 2021 — Remote monitoring of implantable cardiac monitors (ICMs) is highly effective for early detection of ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
September 1, 2021 — University of South Australia researchers have designed a computer vision system that can ...

The annual Healthcare Information Management Systems Society (HIMSS) 2021 meeting is the largest health information ...
August 9, 2021 - In association with Heart Rhythm 2021, Biotronik today announced that the latest implantable cardiac ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
July 29, 2021 – Results from a new clinical trial found human-oversight dependent continuous electrocardiography (ECG) ...
July 29, 2021 — A recent clinical study from Overlake Medical Center utilizing the Bardy Diagnostics Carnation ...
July 27, 2021 — AliveCor Inc., which offers FDA-cleared, smart-phone enabled personal electrocardiogram (ECG) technology ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...

Cardiovascular diseases are among the leading causes of death for people over 65 years old in North America and Europe ...
June 13, 2021 — Medtronic announced clinical trial results from the STROKE AF trial demonstrating the superiority of the ...
July 1, 2021 — The U.S. Food and Drug Administration (FDA) has cleared the Angel Medical Systems Inc. second-generation ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
June 22, 2021 — It is no secret that the use of telehealth technologies rose dramatically with the onset of the COVID-19 ...
June 2, 2021 — Implicity, a leader in remote patient monitoring software and cardiac data management solutions ...
May 10, 2021 – Today, the Consumer Technology Association (CTA) announced it is collaborating with the American College ...


 September 28, 2021
September 28, 2021