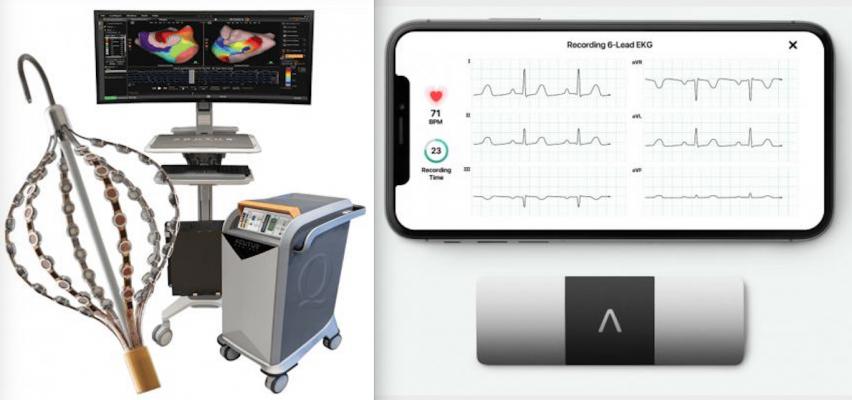
The AcQMap 3D Imaging and Mapping System, left, and the AliveCor KardiaMobile device and app for personal ECG monitoring using a smartphone, right. The companies plan to use remote monitoring to see if it helps improve care for ablation patients.
July 27, 2021 — AliveCor Inc., which offers FDA-cleared, smart-phone enabled personal electrocardiogram (ECG) technology and services, and Acutus Medical, an electrophysiology (EP) lab arrhythmia mapping company, announced a collaboration to assess the integration of data collection tools across the cardiology continuum of care that could potentially advance the field of arrhythmia treatment and disease management.
The management and treatment of complex cardiac arrhythmia patients continues to be a challenge for physicians and global healthcare systems and providers. To address these challenges, Acutus and AliveCor plan to launch in the third quarter of this year a post-market pilot study in the U.S. to gain insights on how physicians can utilize the KardiaMobile device to facilitate pre-ablation and/or post-ablation remote monitoring, which may help guide decision making in the management of cardiac arrhythmia patients. This information will enable more informed decisions in the treatment of complex arrhythmias to potentially improve patient outcomes.
"Utilizing data from across the continuum of care to complement our core mapping and therapy technologies furthers our vision to become the provider-of-choice and democratize the treatment of complex arrhythmia patients," said Vince Burgess, president and CEO of Acutus. "AliveCor will be an excellent partner to help our physicians assess important clinical patient information as they manage and treat their cardiac arrhythmia patients."
Acutus offers a broad and innovative portfolio of highly differentiated electrophysiology tools and products that provide a complete solution for catheter-based treatment of cardiac arrhythmias. The company's core technology is the AcQMap 3D Imaging and Mapping System, the only noncontact mapping system in the world to enable the creation of full chamber global activation maps in real-time.
"AliveCor is proud to partner with Acutus to advance understanding of the use of our shared technologies and how we can potentially improve the care and monitoring of cardiac patients," said Priya Abani, CEO, AliveCor.
AliveCor's FDA-cleared KardiaMobile device, the most clinically validated personal ECG solution in the world, has been used by more than one million people. The KardiaMobile 6L device is the first and only six-lead personal ECG that detects more arrhythmias than any other personal ECG device. KardiaMobile 6L provides instant detection of atrial fibrillation, bradycardia, tachycardia, sinus rhythm with supraventricular ectopy, sinus rhythm with premature ventricular contractions, sinus rhythm with wide QRS, and normal heart rhythm in an ECG. The FDA recently cleared the KardiaMobile 6L device for healthcare professionals to use the device to calculate patients' QTc interval. The acquisition this year of CardioLabs, a leading monitoring and cardiac diagnostic service provider, furthers the mission of AliveCor to extend its comprehensive cardiological services to patients who are recommended monitoring devices by their healthcare providers.
For more information: alivecor.com, www.acutusmedical.com


 February 06, 2026
February 06, 2026 









